Abstract
BACKGROUND AND PURPOSE: Clinicians are insecure reading CT scans by using the one-third rule for acute middle cerebral artery stroke (1/3 MCA rule) before treating patients with recombinant tissue plasminogen activator. The 1/3 MCA rule is a poorly defined volumetric estimate of the size of cerebral infarction of the MCA. A 10-point quantitative topographic CT scan score, the Alberta Stroke Program Early CT Score (ASPECTS), is described and illustrated. A sharp increase in dependence and death occurs with an ASPECTS of 7 or less. We describe how to use ASPECTS and why it works with CT scans obtained on all commonly used axial baselines. We also describe interobserver reliability among clinicians from different specialties and with different experience in reading CT scans in the context of acute stroke.
METHODS: The six physicians who developed ASPECTS answered a questionnaire on precisely how they interpret and use ASPECTS. The ASPECTS areas as interpreted by these physicians were compared with one another and with standards in the literature. κ statistics were used to assess the interobserver reliability of ASPECTS versus the 1/3 MCA rule.
RESULTS: The exact methods of interpretation varied among the six individual observers, with either a 3:3 or 4:2 split on the specific questions. The overall interobserver agreement was good compared with that of the 1/3 MCA rule. Normal anatomic vascular and interobserver variations explain why ASPECTS can be applied with different CT axial baselines.
CONCLUSION: ASPECTS is a systematic, robust, and practical method that can be applied to different axial baselines. Clinician agreement is superior to that of the 1/3 MCA rule.
Intravenous recombinant tissue plasminogen activator (tPA) improves outcome after acute ischemic stroke (1). The European Cooperative Acute Stroke Study (ECASS) trials pioneered the importance of assessing early CT ischemic changes to predict the benefit from intravenous thrombolysis (2, 3). In both trials, the randomization decision depended on whether more or less than one third of the territory of the middle cerebral artery (MCA) was involved. Unfortunately, subsequent research has shown that even experienced stroke physicians and radiologists have difficulty recognizing and quantifying these changes (4–8).
The Alberta stroke program early CT score (ASPECTS) was developed to offer the reliability and utility of a standard CT examination with a reproducible grading system to assess early ischemic changes (<3 hours from symptom onset) on pretreatment CT studies in patients with acute ischemic stroke of the anterior circulation (9). This CT score is simple and reliable and identifies stroke patients unlikely to make an independent recovery despite thrombolytic treatment. Two hundred three consecutive patients with ischemic stroke were treated with intravenous alteplase within 3 hours of onset, and their pretreatment CT scans were scored prospectively. The score divides the MCA territory into 10 regions of interest. ASPECTS is, therefore, a topographic scoring system applying a quantitative approach that does not ask physicians to estimate volumes from two-dimensional images. Ischemic changes on the baseline CT study were seen in 117 (75%) of 156 treated patients (23 had ischemia in the posterior circulation and 24 were treated outside the protocol). The baseline ASPECTS value correlated inversely with the severity of the stroke on the National Institute of Health Stroke Scale (NIHSS) (Spearman's ρ = −.56, P < .001). The baseline ASPECTS value predicted functional outcome and symptomatic intracerebral hemorrhage (P < .001 and P = .012, respectively). The sensitivity of ASPECTS for functional outcome was .78 and specificity was .96; the respective values for symptomatic intracerebral hemorrhage were .90 and .61. A sharp increase in dependent or fatal outcomes occurred with ASPECTS of 7 of less. The inter- and intraobserver reliability of ASPECTS was good to excellent (κ = .71–.89) and consistently superior to the 1/3 rule between observer pairs from the same specialty.
In this article, we explain how ASPECTS is used in practice, illustrate the method with examples, define landmarks and definitions, show why it can be used on CT scans obtained on all commonly used axial baselines, and emphasize that clinicians from different specialties and with varying levels of experience in assessing acute ischemic CT changes can agree on an acceptable level.
Methods
Patients were recruited from both Calgary and Houston, TX. For the purposes of the current analysis, technical information was not available from CT scans obtained in Houston (n = 70). CT scans obtained in Calgary (n = 86) were performed on fourth-generation helical scanners. For better definition, individual scans were acquired using contiguous axial 6-mm sections from the foramen magnum to the suprasellar region and 10-mm contiguous sections through the remainder of the brain. One sixth of the CT scans were obtained using the orbitomeatal line (OML), one third using the superior OML, and half using the inferior OML. Scanner settings were kV = 120, mAs = 400, and mA = 200. The section time was 2 seconds, the matrix size was 512, and a small focal spot with an algorithm was used to reduce bone artifacts and give finer granularity. The mA was reduced near the vertex to 150, 130, and 120 mA for the upper three slices, respectively. Photography was done at a window level of 30 H with a window width of 75 H. All the CT scans were interpreted from film. The areas studied were in the part of the brain cut with 10-mm sections. Images were read in a darkened room, and were studied from near and far and even obliquely.
The interpreters used the ASPECTS method to read the CT scans. The ASPECTS was determined from two standardized axial CT cuts (Fig 1), one at the level of the thalamus and basal ganglion and one adjacent to the most superior margin of the ganglionic structures, such that they were not seen. On these two sections, which were, by definition, not continuous, the MCA territory was allotted 10 points. A single point was subtracted for an area of early ischemic change, such as focal swelling or parenchymal hypoattenuation, for each of the defined regions. A normal CT scan received an ASPECTS of 10 points. A score of zero indicated diffuse ischemic involvement throughout the MCA territory (Figs 2–4). Parenchymal hypoattenuation was defined as a region of abnormally decreased attenuation of brain structures relative to attenuation of other parts of the same structures or of the contralateral hemisphere. Focal brain swelling or mass effect was defined as any focal narrowing of the CSF space due to compression by adjacent structures, such as effacement of cortical sulci or ventricular compression (10).
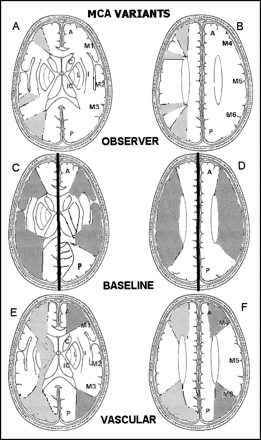
ASPECTS study form and MCA variants.
A and B, Right hemisphere, observer variations: lower and upper ASPECTS slices show as shaded areas the minimal and maximal variations in size of the cortical areas of the MCA (M1–M6) chosen by six expert observers. Left hemisphere, ASPECTS study form: A = anterior circulation; P = posterior circulation; C = caudate head; L = lentiform nucleus; IC = internal capsule; I = insular ribbon; MCA = middle cerebral artery; M1 = anterior MCA cortex; M2 = MCA cortex lateral to insular ribbon; M3 = posterior MCA cortex; M4, M5, and M6 are anterior, lateral, and posterior MCA territories, respectively, approximately 2 cm superior to M1, M2, and M3, respectively, rostral to basal ganglia.
C and D, Cortical MCA area variations with change of baseline. In the right hemisphere, the baseline is parallel to the inferior OML; in the left hemisphere, the baseline is the superior OML.
E and F, Normal vascular variations in MCA size on the two ASPECTS slices. The right hemisphere shows the larger normal variations described by van der Zwan (18) (light shading). The left hemisphere of each shows the smaller, textbook (17), variations (dark shading).
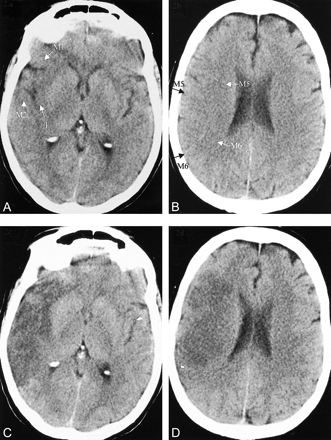
CT scans in a 65-year-old woman with left-sided hemiplegia, hemianopia, and neglect less than 3 hours after symptom onset.
A and B, Baseline CT scans show hypoattenuation with swelling and effacement in regions M1, M2, insula (I), M4, and M5 (ASPECTS = 5). Intravenous thrombolysis was administered.
C and D, Follow-up CT scans show a large area of hypoattenuation involving much of the MCA territory. The patient was dependent at 3 months.
Three neurologists specializing in stroke and three neuroradiologists involved in ASPECTS were interviewed and completed a questionnaire concerning their individual methods of interpretation (Table 1). They were also asked to draw the cortical areas M1 to M6 on the ASPECTS diagrammatic cuts (Fig 1). In addition, they were asked how they differentiated old from new infarcts and whether they used any special techniques for interpretation. The neurologists and the neuroradiologists had at least 2 years' experience in assessing acute ischemic stroke with CT scans. Although radiology trainees participated in the validation of ASPECTS (9), they did not participate in the questionnaire.
Methods used to avoid overreading CT scans of infarcts less than 3 hours old
The questionnaire, devised by a neuroradiologist who had no prior experience with ASPECTS, was developed to assist physicians who were inexperienced in the precise use of this technique. Because interobserver agreement in the original ASPECTS was good to excellent, it was hypothesized that all respondents would give similar answers.
Recent textbooks state that ischemic stroke can be seen as early as 6 hours after symptom onset (11, 12), quoting the abstracts from the classic articles of Tomura et al in 1988 (13) and Truwit et al in 1990 (14). However, the former group made a diagnosis in less than 3 hours in 13 of 15 patients with obscuration of the lentiform nucleus (from a total of 25 patients), while the latter made it in 10 of 13 patients with loss of the insular ribbon (from a total of 27 patients). These descriptions formed the basis for diagnosis in two of the areas of ASPECTS. However, certain considerations remain; such as, did one lose a point if only a small part of the insular ribbon was involved? The questionnaire also sought to answer how many of the signs of ischemia (mass effect, abnormally low attenuation in white matter, loss of demarcation of the gray/white junction) need to be present to lose a point in any area. Furthermore, where were the boundaries of each M area, and did the interpreters really only look at two CT sections?
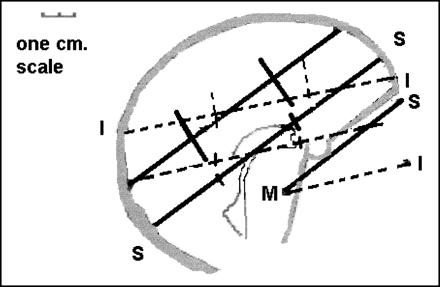
The two extreme axial baselines, the superior OML and the inferior OML, were drawn on an outline traced from a lateral pilot film. The two ASPECTS cuts were then drawn in correspondence with each baseline, and these cut lines were marked in thirds. It was then possible to measure the distances between the two anterior third and the two posterior third junctions, respectively, by using the scale on the pilot scan (Fig 5).
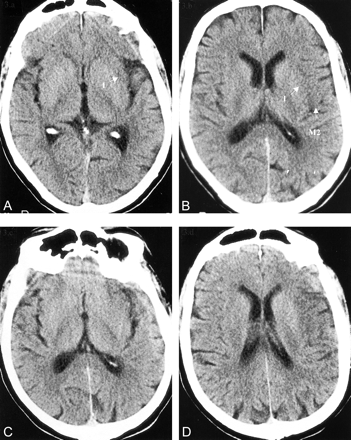
CT scans in a 68-year-old man with global aphasia and an NIHSS score of 7.
A and B, Baseline scans show a region of hypoattenuation involving the anterior insula (I) and hypoattenuation and swelling in the M2 region (ASPECTS = 8).
C and D, Follow-up scans confirm the area of infarction. The patient made a full neurologic recovery.
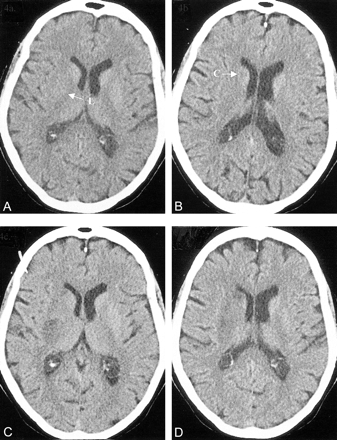
CT scans in a 79-year-old woman with left-sided weakness and NIHSS score of 15, 2 hours after symptom onset.
A and B, Baseline CT scans show hypoattenuation of the right lentiform nucleus (L) and caudate nucleus (C) on two axial cuts (ASPECTS = 8).
C and D, Follow-up scans at 24 hours confirm the area of infarction. The patient made a full neurologic recovery after thrombolysis.
Maximal variation of ASPECTS sections with baseline alteration. The two ASPECTS sections with two different baselines: superior OML (solid line) and parallel two slices, and inferior OML (dashed line) and parallel two slices. The respective upper and lower slices are divided into thirds. Cuts are through the basal ganglia and roof of the lateral ventricle to show that disagreement is not more than 2 cm
We hypothesized that ASPECTS would provide a more standardized assessment of acute ischemic change than the 1/3 rule. The CT scans were interpreted by neurologists specializing in stroke, by neuroradiologists, and by radiology residents individually and in isolation from the others, first with no clinical information and then only with knowledge of which side was affected. A minimum of 3 weeks elapsed between the readings. A sample of convenience of stroke patient CT scans from Calgary (n = 68) was chosen to assess interspecialty reliability. Only the results given with knowledge of the affected side are presented, since these are most relevant to the acute clinical scenario. Interobserver reliability for ASPECTS was assessed according to divisions of greater than 7 and 7 or less, and for MCA territories of less than one third and greater than one third.
Results
How Different Physicians Interpreted ASPECTS (Table 1)
In the lentiform, caudate, and insular regions, all observers agreed upon several points. Hypoattenuation of the insular ribbon was defined as loss of differential density between subcortical white and gray matter of the insular cortex (loss of gray/white matter differentiation). It was emphasized that either the anterior or posterior halves may be lost independently. Obscuration of the lentiform nucleus was defined as a decrease in density of all or part of this nucleus and loss of gray/white matter differentiation in the area. Again, only part of the caudate head had be to obscured or show hypoattenuation to lose a point. The caudate head often has a dual blood supply, from the MCA and anterior cerebral artery (Fig 1E). Comparison was always made with the opposite hemisphere. Loss of a well-defined lateral margin of the lentiform nucleus in both hemispheres raised the differential diagnosis of a CT scan of poor quality, new bilateral infarcts, or one new infarct along with a contralateral old infarct (most likely). All viewers subjectively interpreted a lesion with very marked hypoattenuation as an old lesion. Workstations were not used.
In the M1 to M6 territories, all neuroradiologists required abnormal hypoattenuation plus gray/white margin blurring and/or mass effect before they scored a region as abnormal, but two of three neurologists required only one of these three criteria to subtract a point. One neuroradiologist and two neurologists would call an M area on the basis of mass effect alone, the others additionally required hypoattenuation to be present. All viewers said that they divided the MCA territory roughly into thirds at M4 to M6 levels. All neurologists favored diverging superior and inferior margins for M5, while two radiologists described parallel margins. It was stressed that the M5 territory extends medially to the margins of the lateral ventricle. We are dealing with geometric not anatomic areas. All observers regarded M1 as anterior to the anterior end of the sylvian fissure and included the frontal operculum. All observers identified the anterior end of the temporal lobe as the anterior boundary of M2, but the oblique posterior boundary varied.
There was at least a centimeter difference in the size of each M area between the minimum and maximum chosen by the observers (Fig 1A and B, right hemisphere). For comparison, the right hemisphere of Figure 1C and D shows the usual MCA territory when the inferior OML is used (15) while the left hemisphere shows it using the superior OML baseline (16). Figure 1E and F shows cross-sectional representations of normal variations in MCA size derived from textbooks (17) in the left hemisphere and from van der Zwan (18) in the right hemisphere.
The internal capsule was scored variably. Two neurologists and one neuroradiologist assessed only the posterior limb of the internal capsule; the others assessed both limbs and deducted a point if any portion of it was affected. In their overall assessment, the observers disagreed on several points. Four of the six chose to score the CT scans without clinical information and then reread them knowing which hemisphere was affected. This was a very deliberate attempt to avoid overreading. If one could not decide which hemisphere was involved when one read the scan without any clinical information, the ASPECTS was judged to be greater than 8. All neuroradiologists looked at all CT scans, whereas two of the three neurologists reviewed only the two critical ASPECTS cuts. The neuroradiologists used this assessment to judge whether there was volume averaging or an infarct present, whereas the neurologists looked at either the opposite side or at adjacent sections to make this decision. Two neuroradiologists were slightly more conservative and one stroke neurologist was more aggressive at interpreting the CT scans than the other three.
Very obvious areas of hypoattenuation were interpreted by all observers as old lesions, but this decision was purely qualitative and based both on the concept that very definite hypoattenuation does not occur within 3 hours of stroke onset and on individual experience. Viewing from different angles and distances helped in the recognition of subtle changes in hypoattenuation. In particular, it proved useful to view a crucial slice independently. This was done by using either the cupped hand or a roll of paper (telescoping). Localized atrophy, defined as sulcal widening or ventricular enlargement, also suggested an old infarct. When old infarcts were present in the basal ganglia of the asymptomatic hemisphere, it was sometimes difficult (and occasionally impossible) to say whether areas of abnormal hypoattenuation or obscuration in the basal ganglia of the affected hemisphere represented new or old infarcts.
When a CT scan showed that a patient's head was not symmetrically situated in the scanner, owing to either tilt or rotation in either direction, or both, all observers dealt with this as best they could by trying to compare corresponding areas in the two hemispheres, even though these were on different CT sections.
Because of varying baselines, as much as a 1-cm difference in the rostrocaudal-anteroposterior location of a given point occurred when different cuts were used through the basal ganglia; and as much as a 2-cm difference occurred on the cuts at the upper edge of the lateral ventricles above the basal ganglia (Fig 5).
Interobserver Agreement
Table 2 gives the balanced κ scores for interobserver agreement among all the independent observers when they used the 1/3 MCA rule and ASPECTS dichotomized between 7 or greater and less than 7. The interobserver agreement between pairs of stroke neurologists (κ = .61 for the 1/3 MCA rule and .85 for ASPECTS), neuroradiologists (κ = .52 and .89), and radiology residents (κ = .64 and .71) was reported previously (9). The remainder of the table shows that interobserver agreement across specialties was clearly superior for ASPECTS (κ = .56–.83) over the 1/3 MCA rule (κ = .20–.51). Only one of the 12 results showed less than good reliability for ASPECTS, while all 11 of the 1/3 MCA rule results showed just fair reliability, with the remaining one implying poor reliability.
Pairwise interobserver agreement between independent observers for the 1/3 MCA territory rule and ASPECTS, with knowledge of the affected side
Discussion
Early ischemic changes seen on CT scans obtained in the first few hours after stroke onset represent early cytotoxic edema and perhaps the development of irreversible injury (19). Many authors have cited the potential superiority of diffusion-weighted MR imaging over CT, but to date MR imaging has not been able to discriminate salvageable brain tissue from that which is irretrievably injured (20). Although diffusion-weighted imaging may become the method of choice, most physicians treating stroke will remain dependent on CT because of its accessibility. However, the ability of physicians to correctly interpret early radiologic signs of acute stroke on CT scans is fraught with reservations and controversy (7). We believe that ASPECTS provides a solution to this problem.
ASPECTS is a robust clinical tool for several reasons. First, it has excellent reliability in the clinical setting, much superior to the 1/3 MCA territory rule. When the clinical situation is known, ASPECTS has proved reliable among physicians of different clinical backgrounds and experience. The agreement among physicians using ASPECTS was considerably better than when they applied the 1/3 MCA rule (the range of κ improved from .20–.64 to .56–.89). Acute stroke therapy requires that the treating clinician be comfortable in making an assessment of the severity of the CT findings at the bedside and that communication between colleagues is consistent. We have shown that agreement between neuroradiologists and stroke neurologists was good (κ = .61–.71). This is essential both in facilitating the treatment process and in conducting clinical trials. Analysis of ECASS-1 CT scans found that 52 (8.4%) of 620 were misread locally. When three expert neuroradiologists reassessed the ECASS-1 CT scans, scoring them according to the 1/3 MCA rule, the chance adjusted pair-wise agreement was surprisingly low (κ = .23–.51) despite 90% to 91% agreement (4). In a review of 50 CT scans from the Atlantis study (which used the 1/3 MCA rule) (21), agreement among the three neuroradiologist reviewers was moderate to good (pair-wise κ coefficients of .44–.65) but consensus among reviewers could be achieved in 72% of cases. However, only neuroradiologists interpreted the CT scans in the ECASS and Atlantis studies, and the scans were obtained within 6 hours of ictus. Second, ASPECTS is a systematic method. Wardlaw and Seller (22) showed that a systematic approach to assessing cerebral infarcts on CT scans produces excellent results. In that study, the κ statistics between two experienced neuroradiologists reading 119 brain CT scans for site and size without clinical information were good to excellent (κ = .69–.87) (22). Our analysis showed that good to excellent reliability can be achieved with ASPECTS when the stroke symptom side is known, despite variations in the exact interpretation of the signs of early ischemia and in the use of the two ASPECTS diagrams (Fig 1A and B). One must conclude, therefore, that the improvement stems from careful study of 10 specific areas on the initial CT scan, in which each area is compared with the opposite side.
It is interesting that the instructions for ASPECTS were interpreted differently by the neurologists and the neuroradiologists. Why does ASPECTS work when there is disparity concerning its exact interpretation? First, the two neurologists, who used only two ASPECTS sections to assess a score, missed only isolated infarcts near the vertex, and these by themselves are relatively rare. This would not have much impact on the statistics of 156 CT scans. The fact that one neurologist and one neuroradiologist initially read the CT scans blinded to clinical information reflects the speed and confidence with which these individuals worked. None of the three neurologists distinguished between infarcts and volume averaging by reviewing the whole scan, as did the neuroradiologists, but the neurologists still made limited comparisons, which apparently helped them.
Second, within the narrow concept of using ASPECTS to assess the extent of MCA ischemia, the two dissenting stroke neurologists used only two ASPECTS slices. However, in clinical practice, they would be contemplating giving a powerful thrombolytic drug, tPA, and they had already, in the research protocol, assessed the CT scan using the 1/3 MCA rule. They must, therefore, have read the whole CT scan, if only as a gestalt. The initial use of a CT scan is to exclude hemorrhage and tumor, and, in this context, to look for venous thrombosis.
Six observers were equally divided on the interpretation of what constituted the internal capsule for the purpose of ASPECTS. Surprisingly, this did not make as big an impact on the score as one would have expected. A possible explanation relates to the dichotomization at greater than 7 and 7 or less. Often, the caudate head, lentiform nucleus, and insula are infarcted at the same time, and so a maximum ASPECTS score would be 7, depending on how many, if any, cortical M areas were affected. Half our observers would give it an ASPECTS of 7 or less. Those who deducted a point for the involvement of the anterior half of the internal capsule would score it 6. Because of the level of dichotomy, this would not affect the results. Questions 4 and 5 seem to indicate that in assessing the cortical M areas the precise combination of signs of ischemia is not significant. The important thing is that one look carefully at all areas. Eyeballing, or the gestalt method, of reading a CT scan does not work for the more subtle changes seen in acute infarcts less than 3 hours from onset.
Third, ASPECTS retains its utility with different CT techniques. Techniques have been fully discussed by Graeb (23) and Russell (24), and optimal ones suggested, particularly the use of a large mAs. Lev et al (25) have discussed the benefit of workstations, showing that the detection of ischemic brain parenchyma is facilitated with variable window widths and center settings to accentuate the contrast between normal and edematous tissue. These authors initially used a center of 32 H with a width of 8 H. Reviewers then changed the settings to accentuate differences. Use of a narrow window width to review the CT scan on the workstation should improve the detection of early acute infarcts, just as it facilitated the diagnosis of isoattenuating subdural hematomas over a decade ago. The design of the original ASPECTS study (9) did not incorporate the use of workstations.
The ASPECTS system was used with three different CT scan baselines. Early CT scans of the head were obtained with a superior OML baseline, but some centers have now changed to an OML or an inferior OML. There was at least a 1-cm difference in the size of each M area between the minimum and maximum chosen by each of our observers; normal variations in the size of the MCA territory are probably greater than this (18). On the ganglionic level, the anatomic division of sections by the observers was always based on the position of the ends of the sylvian fissure. Therefore, a change of baseline would have no significant effect on the interpretation here. At the level of the roof of the ventricles, the baseline changes made a rostrocaudal-anteroposterior difference of 2 cm in the location of the M4 to M6 regions. The three variable factors are variations in observer interpretations, baseline variations, and vascular anatomic variations. As the latter is the most variable, we postulate this is the reason that ASPECTS can be used successfully on scans obtained on all axial baselines. This may not apply to the 1/3 MCA territory rule.
Conclusion
The availability and speed of CT scanners make them the instrument of choice for assessing acute ischemic stroke in many hospitals. While acute MR imaging provides fantastic pathophysiological information, the utility and widespread applicability of diffusion-weighted MR imaging has yet to be proved within the first 3 hours of stroke ictus. ASPECTS is a CT-based system that provides a more accurate, robust, and practical method for assessing acute ischemic stroke than the 1/3 MCA rule. We encourage clinicians and radiologists to apply it in practice.
Footnotes
1 Supported in part by grants from the Heart & Stroke Foundation of Canada (M.D.H., A.M.B.), the Canadian Institutes of Health Research (M.D.H., A.M.D., A.M.B.), and the Alberta Heritage Foundation for Medical Research (M.D.H., A.M.D., A.M.B.).
2 Address reprint requests to Professor J. H. Warwick Pexman, Department of Clinical Neurosciences, Room MRG005, Foothills Hospital, 1403 29th St NW, Calgary, Alberta, Canada T2N 2T9.
References
- Received November 2, 2000.
- Accepted after revision March 27, 2001.
- Copyright © American Society of Neuroradiology