Abstract
Summary: Absence of the internal carotid artery (ICA) is a rare congenital anomaly. The embryology of the ICA and the common collateral pathways associated with its congenital absence are reviewed, with four new cases provided for illustration. While collateral blood flow may allow these patients to remain asymptomatic, two of our patients presented with transient ischemic attacks. Recognition of this anomaly has important implications during planned carotid or transsphenoidal surgery, in thromboembolic disease, and in the surveillance and detection of associated cerebral aneurysms.
Agenesis, aplasia, and hypoplasia of the internal carotid artery (ICA) are rare congenital anomalies, occurring in less than 0.01% of the population (1, 2). The term absence has been chosen to incorporate agenesis, aplasia, and hypoplasia of the ICA. In this setting, the most common type of collateral flow is through the circle of Willis. Less commonly, collateral flow is provided via persistent embryonic vessels or from transcranial collaterals originating from the external carotid artery (ECA) system. Slightly more than 100 cases of congenital absence of the ICA have been reported in the literature (3). We report four new cases of absence of the ICA, including one with an associated intercavernous anastomosis.
Case Reports
Case 1
A 56-year-old man presented with transient right-sided weakness and numbness. Sonographic evaluation of the carotid bifurcation (not shown) revealed a diffuse narrowing of the left ICA along its entire visualized course, with a markedly decreased peak systolic velocity of 20 cm/s. The contralateral side was remarkable for only mildly increased flow volume within the right common carotid artery (CCA). On the basis of the sonogram, a congenitally small left ICA was suspected, but a chronic dissection or severe atherosclerosis could not be excluded. The patient subsequently underwent MR imaging of the circle of Willis, which showed attenuated flow within the petrous portion of the left ICA (Fig 1A) and collateral flow to the left hemisphere through the circle of Willis (Fig 1B). Hypoplasia of the left carotid canal was discovered at CT angiography (Fig 1C), which confirmed the congenital nature of the small left ICA. The patient's symptoms resolved spontaneously and were attributed to either transient ischemic attacks or migraine headaches. No thromboembolic source was identified.
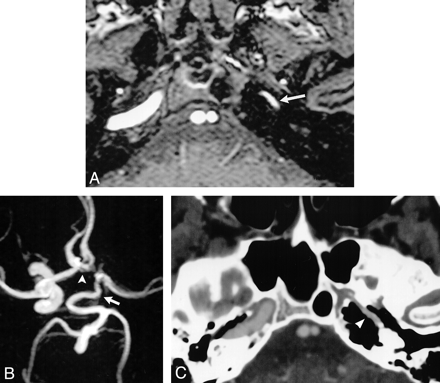
Case 1: Hypoplasia of the left ICA.
A, Source image from a 3D time-of-flight MR angiogram shows diminished flow-related signal intensity within the petrous portions of the left ICA (arrow).
B, Compressed image from the MR angiogram shows a tortuous, enlarged PCOM (arrow) extending forward to supply the left MCA. The left ACA is supplied via a patent ACOM (arrowhead). There is no perceivable flow-related signal intensity within the supraclinoid left ICA on the compressed image.
C, Axial image from a CT angiogram at the level of the petrous ICA shows hypoplasia of the left carotid canal (arrowhead).
Case 2
A 6-year-old girl presented with a single episode of slurred speech and headache, followed by drowsiness. Her symptoms resolved after approximately 10 minutes and she experienced neither loss of consciousness nor loss of bowel or bladder control. A diagnosis of simple partial seizure disorder was made, but an incidental finding of unilateral ICA agenesis was discovered at MR imaging. The MR angiogram centered at the circle of Willis revealed absence of flow-related signal intensity within the left supraclinoid ICA (Fig 2A), with collateral flow to the left anterior cerebral (ACA) and middle cerebral (MCA) arteries via a patent anterior communicating artery (ACOM) (Fig 2B). Absence of the carotid canal on a skull base CT scan (Fig 2C) confirmed agenesis of the left ICA. Clinical concern for Moya-Moya disease led to the performance of cerebral angiography, which revealed a small-caliber left CCA terminating in the ECA, and collateral flow to the left cerebral hemisphere across a patent ACOM (Fig 2D). Stenosis of the A1 segment of the right ACA (Fig 2B and D) is of uncertain pathogenesis, but has been shown to be of no clinical significance over a several year follow-up period, and the patient has remained asymptomatic with pharmacologic management of her seizure disorder.
Case 2: Agenesis of the left ICA.
A, Source image from a 3D time-of-flight MR angiogram reveals absence of flow-related signal within the left petrous ICA (arrow).
B, Compressed view from the MR angiogram displays absence of flow-related signal intensity within the left ICA with collateral supply to the left hemisphere through a patent ACOM. Normal flow is present within the right ICA (arrowhead). Focal loss of flow-related signal intensity within the A1 segment of the right ACA (arrow) represents a clinically insignificant stenosis.
C, Axial CT scan of the skull base shows absence of the left carotid canal and a normally developed right carotid canal (arrow).
D, Collateral supply to the left cerebral hemisphere is provided through a patent ACOM, as demonstrated on the frontal view from the right carotid arteriogram. Stenosis of the A1 segment of the right ACA is revealed again (arrow).
Case 3
A 62-year-old man presented for evaluation of transient ischemic attacks. MR imaging showed an abnormal right cavernous carotid flow void (not shown), which prompted further evaluation with cerebral angiography. The arteriogram revealed a right CCA of diminished caliber that terminated in the ECA, with no remnant of a cervical right ICA. The right ACA was supplied through a patent ACOM, and the right MCA was a continuation of the right supraclinoid ICA (Fig 3A). The right carotid siphon received blood flow through an intercavernous collateral vessel arising from the left ICA and coursing through the sella turcica (Fig 3A and B). The intercavernous communication confirmed a congenitally absent right ICA, but agenesis could not be discerned from aplasia, as a CT examination was not available for evaluation of the skull base (see Discussion).
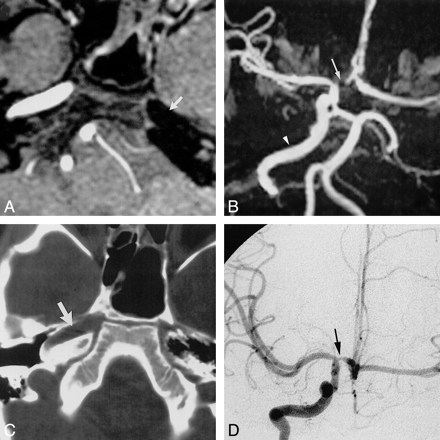
Case 3: Absence of the right ICA.
A and B, Frontal (A) and lateral (B) projections from a left CCA arteriogram show an anomalous communication between the cavernous portions of the ICAs (arrow). This anomalous communication courses through the sella turcica. The right ACA is supplied via a patent ACOM, with the right A1 segment either being aplastic or extremely hypoplastic. The right MCA is a continuation of the right supraclinoid ICA.
Case 4
A 33-year-old woman experienced sudden onset of left upper extremity weakness 10 days after being involved in a motor vehicle accident. Cerebral angiography revealed a focal dissection of the right cervical ICA. The left CCA terminated into the ECA with no identifiable remnant of the ICA. Collateral flow to the left hemisphere was provided across a patent ACOM (Fig 4A) and through a patent posterior communicating artery (PCOM) (Fig 4B). Review of the patient's head CT examination disclosed a diminutive left carotid canal (Fig 4C), confirming the diagnosis of left ICA aplasia and effectively excluding traumatic dissection or occlusion of the left ICA. The patient sustained concomitant intraabdominal injury, which precluded anticoagulation until several weeks after her exploratory laparotomy.
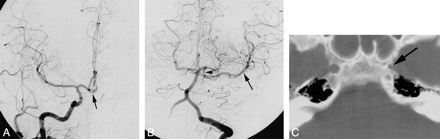
Case 4: Aplasia of the left ICA.
A, Frontal view from a right CCA arteriogram shows collateral flow to the left ACA across a patent ACOM (arrow).
B, Frontal view from a left vertebral arteriogram displays collateral flow to the left MCA (arrow) via forward flow through a patent PCOM (arrowhead).
C, Axial CT scan through the skull base reveals a diminutive left carotid canal (arrow).
Discussion
Tode is credited with the first documented case of carotid agenesis, discovered on postmortem examination in 1787 (4). In 1954, the first case of ICA agenesis at cerebral angiography was reported by Verbiest (3). Lie (5) defined agenesis as complete failure of an organ to develop, aplasia as lack of development (but its precursor did exist at one time), and hypoplasia as incomplete development of the organ (6). Although an exact cause of these developmental anomalies has not been established, all three variations are thought to represent the sequela from an insult to the developing embryo (7). Postulated causes of unilateral absence have centered on mechanical and hemodynamic stresses placed on the embryo, including effects related to exaggerated folding of the embryo toward one side and constriction by amniotic bands (7). To date, an explanation for bilateral absence has not been rendered (7).
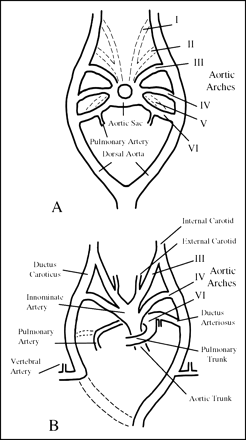
Padget's (8) analysis of the embryo provided useful insight into the development of the ICA, revealing its origin from the dorsal aorta and the third aortic arch at approximately the 4- to 5-mm embryonic stage, with the full development of the ICA by 6 weeks (Fig 5). Although it is generally accepted that the ICA originates from the third aortic arch, the origin of the CCA and ECA remains controversial (3, 5, 6). Some investigators argue that both the proximal ICA and the ECA arise jointly from the third aortic arch, and thus agenesis of the ICA should be accompanied by absence of the ipsilateral ECA. Others argue that the ECA and CCA can develop normally in the setting of ICA agenesis, as the former arises independently from the aortic sac (5, 6). The latter seems more plausible, as numerous cases of ICA agenesis exist in the literature (index cases included) with normally developed ECA systems.
A and B, Illustrations of the developing embryo at 6 mm (A) and 11 mm (B). After Congdon, as reproduced in (5); see text
The circle of Willis forms during the 7- to 24-mm stage of embryonic development (9). In cases of absence of the ICA, the pattern of collateral blood flow to the distal ICA and intracranial vasculature is dependent upon the stage at which the disruption occurred. Cali et al (9) postulated that primitive pathways of collateral circulation (ie, intercavernous anastomoses) would prevail if the disruption occurred before completion of the circle of Willis. Likewise, collateral flow through the circle of Willis would dominate if the disruption occurred after the 24-mm stage of development.
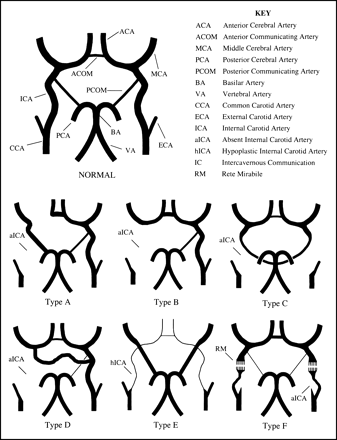
Lie (5) described six pathways of collateral circulation in association with absence of the ICA (Fig 6). In type A (Figs 1B and 4B and C), unilateral absence of the ICA is associated with collateral circulation to the ipsilateral ACA through a patent ACOM and to the ipsilateral MCA from the posterior circulation through a hypertrophied PCOM. In the type B (Fig 2B and D) pattern of collateral flow, the ipsilateral ACA and MCA are supplied across a patent ACOM. Type C represents bilateral agenesis of the ICA with supply to the anterior circulation via carotid-vertebrobasilar anastomoses, and review of the literature indicates this is generally accomplished through hypertrophy of the PCOM (5, 9–12). Type D (Fig 3A) represents unilateral agenesis of the cervical portions of the ICA with an intercavernous communication to the ipsilateral carotid siphon from the contralateral cavernous ICA. In type E, diminutive ACAs are supplied by bilateral hypoplastic ICAs, and the MCAs are supplied by enlarged PCOMs. The type F pattern provides collateral flow to the distal ICA via transcranial anastomoses from the internal maxillary branches of the ECA system, the so-called rete mirabile. Others (2, 13) have simplified Lie's original six collateral pathways into three main types: collateral flow through the circle of Willis (most frequent), collateral flow via persistent fetal circulation, and reconstitution of the ICA through skull base collaterals from the ECA.
Although types A, B, C, and E represent either hypertrophy of normally present embryologic vessels or their persistence, an in utero communication between the ICAs has not been observed to explain Lie's type D intercavernous communication (5, 6). Two prevailing theories exist on the origin of this intercavernous communication. In the first, the collateral vessel is thought to arise from a union between two primitive trigeminal arteries that have lost their communication with the basilar artery (5). The second proposes hypertrophy of embryologically developing vessels, such as the plexiform network of vessels surrounding Rathke's pouch described by Padget (8, 14) or remnants of the primitive maxillary artery, to account for the intercavernous communication (5, 13–16). The differing theories surrounding the origin of this communication may stem from its variable course in relation to the sella turcica. The intercavernous collateral vessel may be located posterior to the clivus, or run above, through, or in the floor of the sella turcica (17). At imaging, the transsellar variety (Fig 3A and B) of this vessel has been known to mimic a pituitary microadenoma (18).
In Lie's original illustration of the type D pattern of collateral flow (and subsequent adaptations), supply to the ipsilateral ACA and MCA is derived from both the intercavernous communication and a patent ACOM (5, 6). These illustrations differ slightly from the reported cases of this intercavernous vessel (2, 4–6, 14, 16–18). When an intercavernous communication exists, there is associated hypoplasia (if not aplasia) of the ipsilateral A1 segment of the ACA with the patent ACOM supplying only the ipsilateral distal ACA territory (Fig 3A). Thus, in the type D pattern of collateral flow, the majority (if not all) of the ipsilateral MCA supply is derived from the intercavernous communication, essentially rendering the hemisphere isolated from alternate routes of collateral flow (Fig 3A).
Since Lie's description in 1968 (5), a persistent trigeminal artery reconstituting the ICA and MCA in the setting of unilateral agenesis has been reported as an additional pathway of collateral flow (19). While a full discussion of the persistent trigeminal artery (and other persistent carotid-vertebrobasilar anastomoses) is beyond the scope of this review, this is thought to represent the first reported case of anteriorly directed flow from the basilar system to the ICA through a trigeminal artery. All previously reported cases of persistent trigeminal arteries were associated with posteriorly directed collateral flow from the ICA to the basilar system and hypoplasia of the basilar artery (if a hypoplastic vessel were present) (19). Differentiating a hypertrophied PCOM from a persistent trigeminal artery is generally easily accomplished with either MR or CT angiography, as the PCOM's origin from the supraclinoid ICA can be distinguished from the persistent trigeminal artery's origin from the cavernous ICA. However, Wismer (20) reported an unusual case of a variant trigeminal artery that originated from the supraclinoid rather than the cavernous portions of the ICA.
While the literature supports a nearly 3:1 left-sided predominance of ICA absence (7), there is no side predilection when absence of the ICA is associated with an intercavernous anastomosis (4). Congenital absence may be unilateral or bilateral, although the unilateral variety is distinctly more common (9). There may be complete absence of the ICA, or only a portion of the ICA may be missing (type D, Fig 5). A tiny fibrous band may be the only remnant of the ICA in cases of aplasia, and angiography alone may be incapable of differentiating it from agenesis (5). Evaluation of the skull base for the presence or absence of the carotid canal may be required for distinguishing aplasia (Fig 4C) from agenesis (Fig 2C), as presence of the ICA (or its precursor) is a prerequisite for development of the carotid canal at 5 to 6 weeks of gestation. Therefore, demonstrating an absence of the carotid canal with skull base CT will confirm the diagnosis of agenesis (6). Similarly, demonstrating the presence of a diminutive carotid canal with CT (Fig 1C) will permit one to differentiate hypoplasia of the ICA from acquired conditions resulting in a small-caliber ICA. Acquired causes of an ICA of diminished caliber include chronic dissection, fibromuscular dysplasia, and severe atherosclerosis (21). These distinctions have clinical implications, as the most common pattern of collateral flow in cases of acquired occlusion of the ICA (through the ACOM) is similar to the type B pattern seen with unilateral absence.
Relatively few cases of absence of the ICA have been reported in children, suggesting that initially the collateral pathways are sufficient to support cerebral perfusion (5). While many cases of absence of the ICA may remain asymptomatic and go unrecognized, these patients may present later in life with symptoms related to cerebrovascular insufficiency. The entire anterior circulation may be dependent upon a single carotid artery or the vertebrobasilar system, either of which may become compromised by atherosclerosis. Congenital absence of the ICA was discovered in two of our patients (cases 1 and 3) during evaluation of symptoms ultimately attributed to transient ischemic attacks, although no thromboembolic source or documented hypotensive episode was identified in either case. Alternatively, such patients may present with mass effect from the enlarged collaterals, complications related to aneurysm, and, rarely, congenital Horner's syndrome (22). The estimated prevalence of cerebral aneurysms in the general population is 2% to 4%, but the reported prevalence of aneurysms in association with absence of the ICA is 24% to 34% (1, 6). Increased flow through collateral vessels and altered flow dynamics are cited as plausible explanations for this increased prevalence (1, 6). These appear to be likely explanations, as the ACOM (a common collateral vessel) is the most frequent site of aneurysm formation in such cases (1). The increased risk of aneurysm has been listed as an indication for clinical and radiologic surveillance in these patients (6).
Recognition of this anomaly becomes important in thromboembolic disease, as emboli in one cerebral hemisphere may be explained by atherosclerotic disease in the contralateral CCA or vertebrobasilar system. Consideration of this anomaly will be of utmost importance when planning carotid endarterectomy, as both cerebral hemispheres may be dependent upon the atheromatous carotid. Identification of this variant may help prevent the erroneous diagnosis of carotid dissection or high-grade carotid stenosis, as a case of ICA hypoplasia has been mistaken for the angiographic string sign associated with a critical carotid stenosis (21). Heth et al (21) reported a case in which the hypoplastic nature of the ICA was not revealed until exposure at carotid endarterectomy. Finally, failure to recognize the intercavernous collateral can have grave implications during transsphenoidal hypophyseal surgery (18).
Conclusion
Agenesis, aplasia, and hypoplasia of the ICA are rare anomalies. The main collateral pathways recruited in this setting include the circle of Willis, persistence of embryonic vessels, and transcranial collaterals via the ECA. Although many of these cases remain asymptomatic and go undetected, their recognition is of more than trivial interest. In addition to associated cerebral aneurysms, these anomalies have important implications during carotid endarterectomy and transsphenoidal hypophyseal surgery, and in the setting of thromboembolic disease.
Footnotes
1 Cum Laude award winner for scientific exhibit at the annual meeting of the American Society of Neuroradiology, Boston, April 2001.
2 Address reprint requests to Curtis A. Given, II, MD, Department of Neuroradiology, Wake Forest University, 2nd Floor Meads Hall, Baptist Medical Center, Medical Center Blvd, Winston-Salem, NC 27157.
References
- Received March 18, 2001.
- Copyright © American Society of Neuroradiology