Abstract
BACKGROUND AND PURPOSE: Biochemical studies of seizures in patients and laboratory animals have monitored postictal perturbations in cerebral metabolism with either invasive techniques or with such noninvasive techniques as nuclear medicine, MR imaging, in vivo phosphorus MR spectroscopy (MRS), and in vivo proton MRS at field strengths of 1.5 T or above. We investigated postictal metabolic changes in a generalized seizure model with in vivo proton MRS at 0.5 T, in which the combination of glutamate and glutamine resonances (denoted glx) can be modeled as a singlet.
METHODS: Five adult mongrel dogs underwent control and postictal experiments in which single-voxel proton MR spectra were obtained from the right frontal lobe cortex with a point-resolved spectroscopy technique approximately every 20 minutes for 3 hours. N-acetylaspartate (NAA), glx, and creatine (Cr) were quantified in absolute millimolar units with a cerebral water-referenced algorithm. Inter- and intrasubject differences in mean metabolite concentrations collected throughout the 3-hour period were compared using an unpaired, two-tailed Student's t test at a .05 level of significance.
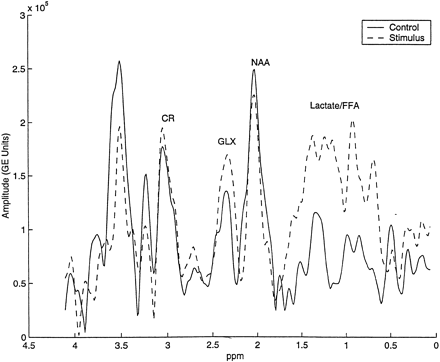
RESULTS: We found a significant increase (15.4%) in the postictal intersubject mean glx concentration, as well as a 23.7% postictal decrease in the intersubject mean Cr concentration. A trend toward a subtle decrease in postictal intersubject mean NAA concentration was not statistically significant. We also observed a substantial qualitative increase in the combination of postictal lactate and free fatty acid peaks.
CONCLUSIONS: The glx, NAA, lactate, and free fatty acid results are in general agreement with previous studies of postictal perturbations in cerebral metabolism measured with invasive biochemical or noninvasive high-field-strength in vivo MRS detection assays. Given a high sensitivity for glx at 0.5 T relative to 1.5 T, further studies of postictal mesial temporal lobe structures are warranted in chronic animal preparations that model temporal lobe epilepsy.
Seizures are a symptom of many different cerebral disorders or syndromes. They may result from an underlying systemic metabolic derangement, such as lactic acidosis, or from primary CNS dysfunction, such as cerebral dysgenesis, meningitis, tumor, and encephalitis (1). Epileptic seizures also produce cerebral and systemic metabolic derangements, some of which may lead to neuronal injury and subsequent necrosis. The cellular and metabolic mechanisms involved in the pathogenesis of neuronal necrosis are hypothesized to originate from the massive neuronal release of excitatory neurotransmitters, such as glutamate, into the synaptic cleft (2).
Previous studies have documented significantly altered glutamate and glutamine (glx) concentrations in ictal and postictal brain samples from laboratory animals and in human epileptic foci after intraoperatively induced, prolonged EEG discharges (3–9). In vitro microdialysis assays have monitored excitatory neurotransmission directly, as these assays can help detect biochemically free (ie, neither part of nor bound to macromolecules) amino acids within intra- or extracellular fluids (10–12). In vitro high-pressure liquid chromatography (HPLC) and analytical nuclear MR imaging (NMR) analysis of perchloric acid extractions of excised brain samples (13–15) have been used to detect biochemically free amino acids involved in neurotransmission and other metabolic pathways, including amino acid synthesis and the production of cellular fuel via the tricarboxylic acid (TCA) cycle (16). Similarly, other studies of seizures in laboratory animals have documented postictal shifts in the creatine/phosphocreatine (Cr/PCr) equilibrium favoring adenosine triphosphate (ATP) synthesis, as measured by classic biochemical means (14, 17). Previous studies have also demonstrated significant increases in postictal lactate in animals by using in vitro biochemical assays of brain tissue samples (15, 17) as well as in vivo proton MR spectroscopy (MRS) at 1.89 T (18).
With the advent of in vivo MRS and cerebral water-referenced quantification algorithms, it has become possible to measure postictal changes in biophysically free (ie, freely vibrating, rotating, and translating) cerebral metabolites in absolute concentration units (mM). However, current clinical in vivo MRS techniques generally depict biophysically free metabolites without regard to their physiological (ie, intra- vs. extracellular) or subcellular (ie, intravesicular vs. mitochondrial vs. cytosolic) compartmentalization, implied functions, or rates of transcompartmental exchange (19).
In this study, we examined perturbations in cerebral metabolism after chemically induced generalized seizures with in vivo MRS at 0.5 T. The derived metabolite concentrations were compared with results of other clinical and experimental studies of seizures in which classic biochemical assays and in vivo MRS were used for metabolite quantification. Our working hypothesis for future studies, prompted by a recent clinical case report of the use of MRS at 1.5 T in a patient after multiple seizures (20), was that postictal glx would be a useful indicator of focal seizure activity, particularly in regions of the brain that are rich in glutamate receptors, such as the hippocampus. Such an indicator may have particular benefit in patients with chronic temporal lobe epilepsy (TLE) with nonlateralizing findings on interictal MR spectra or other imaging studies (21).
Methods
Animal Preparation
Five adult mongrel dogs (three male, two female; 15–25 kg) were sedated with intramuscular fentanyl, droperidol, and atropine, and infused with thiopental (15–25 mg/kg IV). They were anesthetized with intravenous fentanyl and droperidol (0.4 mg fentanyl plus 20 mg droperidol/mL infused at 0.1 mL/kg per hour) (22). The anesthetic infusion was titrated with additional intravenous and intramuscular administrations of fentanyl, droperidol, and atropine as needed. The animals were intubated for airway protection and mechanically ventilated with supplemental oxygen. The neuromuscular blocking agent pancuronium (0.08 mg/kg IV Q 60–90 minutes) (23) was administered as needed throughout both the control and stimulus experiments to eliminate subject motion (Fig 1). Oxygen delivery and ventilation rates were adjusted to maintain heart rate, blood oxygen saturation (SPO2), and blood pH within normal limits. Heart rate and SPO2 were continuously monitored with MR-compatible equipment (Invivo Research, Inc, Orlando, FL). Arterial blood pH and glucose levels (glucometer; Lifescan, Inc, Ft Washington, PA) were sampled periodically.
A and B, T2-weighted fast spin-echo localizer images show reproducible locations for single-voxel proton MR spectra obtained in the same subject in control (A) and postictal (B) experiments conducted on different days.
C and D, T2-weighted fast spin-echo localizer images in another subject before (C) and 3 hours after (D) PTZ-induced generalized seizures show no evidence of head movement or cerebral edema at the conclusion of a postictal experiment.
Image-Guided Single-Voxel Proton Spectroscopy
Multiple T2-weighted coronal MR images were acquired to localize a single cubic voxel (10.3 × 10.3 × 15 mm) for subsequent spectroscopic acquisition. The voxel was centered in the left frontal lobe cortex, in which scalp fat was avoided and CSF minimized by inspection of localizer images (Fig 1). MRS voxels were placed over the same area of the frontal lobe cortex in both control and stimulus experiments as determined by localizer images. Parameters for spectroscopic acquisition were as follows (24): a point-resolved spectroscopy (PRESS) excitation sequence using two-cycle phase alteration and three chemical shift-selective (CHESS) pulses for water suppression (bandwidth 50 Hz), a TR/TE of 1500/41, an acquisition bandwidth of 1000 Hz, and a 1024-point data array. A total of 256 images were averaged for an acquisition time of 6.4 minutes. Before the acquisition of the solvent-suppressed metabolite data, a spectrum of unsuppressed water was obtained under identical parameters for use as phase and concentration references during postprocessing.
Experimental Protocol
Each animal was examined in control and stimulus (postictal) conditions that were separated by at least 2 days; therefore, each animal acted as its own control. During the control experiments, each of the animals was prepared as described above. Proton spectra were acquired at approximately 20-minute intervals for a period of about 3 hours (Fig 2).
A, A control spectrum obtained from the frontal lobe cortex of an anesthetized, chemically paralyzed, mechanically ventilated mongrel dog with the PRESS (1500/41/256) technique at 0.5 T.
B, Lorentzian-fitted peaks for the NAA, glx, and Cr resonances in A.
C, Superimposition of the control spectrum in A and the lorentzian-fitted peaks in B.
In the stimulus experiments, MRS equipment was tested, and a baseline scalp EEG was obtained before seizure onset. EEG-monitored generalized seizures were induced throughout a 30-minute period with repeated injections of the convulsant pentylenetetrazol (PTZ, 20 mg/kg Q 3–10 minutes) (25). After the ictus, per the control experiments, proton spectra were acquired at approximately 20-minute intervals for a period of about 3 hours.
MRS Postprocessing
In vivo metabolite concentration estimates were calculated according to the cerebral water-referenced method described by Soher et al (26), with a few minor modifications specific to PRESS spectra. Briefly, spectra were corrected for phase errors by multiplying the metabolite data by the complex conjugate of the reference water spectrum (27). After phase correction, spectra were zero-padded to 4096 points and broadened exponentially by 2 Hz. Each resonance peak to be quantified, as well as the unsuppressed reference water spectrum, was fitted to the lorentzian absorption lineshape using a Gauss-Newton nonlinear least squares fitting algorithm (The Mathworks, Inc, Natick, MA). The resulting lorentzian absorption lineshape metabolite fits were then integrated, and the calculated areas beneath the peaks were corrected for relaxation losses according to the PRESS-specific relation 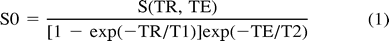 where S0 is the corrected and S the acquired integrated absorption signal.
where S0 is the corrected and S the acquired integrated absorption signal.
T1 metabolite relaxation times of 259, 399, and 473 milliseconds for glx, N-acetylaspartate (NAA), and the brain water reference, respectively, were measured in the frontal lobe cortex of healthy adult human volunteers with an inversion-recovery PRESS technique (unpublished results). For standard PRESS (TR = 1500), the 1 − exp(−TR/T1) correction term in the denominator of Equation 1 of 0.9767 for NAA and 0.9969 for glx were considered negligible. The T1-dependent correction term was assumed to be negligible for Cr at 0.5 T (TR = 1500), since Cr is known to have T1 values comparable to NAA at 1.5 T (28). T2 metabolite lifetimes for NAA, glx, Cr, and brain water of 339, 181, 232, and 123 ms, respectively, were measured in healthy human control subjects with the PRESS sequence (unpublished results). Interspecies differences in relaxation time constants between humans and canines were assumed to be negligible.
Metabolite concentrations were calculated with the equation 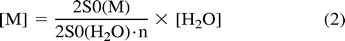 where n is the number of protons contributing to the resonance peak to be quantified. An internal cerebral water concentration of 44.05 M (29) measured from human postmortem material (entire cerebrum) was used, under the assumption that interspecies differences in cerebral water content and distribution were negligible.
where n is the number of protons contributing to the resonance peak to be quantified. An internal cerebral water concentration of 44.05 M (29) measured from human postmortem material (entire cerebrum) was used, under the assumption that interspecies differences in cerebral water content and distribution were negligible.
Lactate, a complex doublet resonance centered at about 1.33 ppm, and free fatty acids, a broad signal with peaks at approximately 0.9 and 1.2 ppm, were not modeled well by the lorentzian lineshape. Therefore, concentration estimates were not calculated for these species. Changes in the lactate plus free fatty acid resonance peaks were observed qualitatively with respect to the NAA resonance.
Physiologically impossible concentration estimates attributed to nonconvergence of the lorentzian fitting algorithm and estimates calculated to be below the detection threshold of MRS (about 1 mM) were excluded. Intra- and intersubject mean postictal changes in metabolite concentrations collected throughout the 3-hour control and postictal experiments were compared with a paired, two-tailed Student's t test. Since a greater number of spectra were usually collected over the 3-hour control condition than the 3-hour stimulus runs, pairing the data required that some technically adequate control spectra be excluded. However, posttesting determined that pairing of the intersubject data was ineffective, so control and postictal experiments were considered independent for practical purposes. An unpaired Student's t test admitted a greater number of control than postictal spectra and generated intersubject results with the same statistical significance as the paired test. The significance level was set at P < .05 (Excel, Microsoft Corp, Redmond, WA; Stata, Stata Press, College Station, TX).
Results
The control experiment for dog 1 was unsuccessful owing to the inadvertent use of a sandbag with a metallic grommet to stabilize the head within the MR detector coil. Dog 1 was excluded from the study and was subsequently used to test the PTZ convulsant doses under anesthesia with fentanyl plus droperidol, the EEG equipment, and the MRS signal strength in dogs.
Response to Stimulus
All animals had generalized seizures, as monitored by EEG, typically lasting 3 to 7 minutes after each PTZ injection. The seizure duration progressively increased with each additional PTZ injection. In all stimulus experiments, generalized seizures were maintained throughout a 30-minute time period with repeated injections of PTZ as needed (range, four to six injections). Marked systemic acidosis was observed in the immediate postictal period. The ventilatory and oxygen delivery rates were adjusted to eliminate systemic acidosis over the period of postictal MRS acquisition so as to mimic supportive measures required by some patients after generalized status epilepticus.
Cerebral Metabolites
A statistically significant increase of 15.4% (postictal vs. control) in the intersubject mean glx concentration estimate was demonstrated after generalized seizures (Table). A significant decrease of 23.7% in the postictal intersubject mean Cr concentration estimate was demonstrated as well. A trend toward a subtle decrease in postictal intersubject mean NAA concentration by 5.6% was not statistically significant. A power analysis (Stata) revealed that to have observed a 5.6% drop in NAA with 5% significance and 80% power, given the observed intersubject mean values, standard deviations, and ratio of stimulus to control spectra listed in the Table, a sample size of 195 control and 142 postictal spectra would have been required.
Control and postictal (stimulus) frontal lobe cortex metabolite concentrations in adult canines
The intrasubject mean postictal glx concentration exceeded the control value in four of five dogs, but reached a statistically significant difference in only one of these four subjects. Similarly, the intrasubject mean Cr concentration was reduced in the postictal period in four of five dogs. The intrasubject difference in Cr reached statistical significance in three of these four animals. The intrasubject mean NAA concentration decreased in the postictal period in four of five dogs, but reached a statistically significant difference in only one of these four subjects.
Although not quantified, a mixture of cerebral lactate (1.15 ppm and 1.5 ppm doublet centered at 1.33 ppm at 0.5 T) and long-chain free fatty acids (broad peaks at 0.9 and 1.3 ppm) that co-resonate within the same broad spectral range, was qualitatively increased in nearly all postictal spectra. Such increases (Fig 3) were observed visually by a comparison of relative amplitudes between overlapping lactate plus free fatty acids and NAA.
A control and a postictal spectrum obtained from the same subject were normalized by the area under the unsuppressed reference water peak and plotted on the same y-axis (arbitrary units). Note the postictal increase in glx amplitude and the emergence of lactate and free fatty acid peaks
Discussion
Combined Glutamate and Glutamine
At 0.5 T, the glx resonance represents the sum of glutamate and glutamine resulting from a collapse of the γ and β multiplet resonances (24). Using the cerebral water-referenced method and assuming a singlet lineshape for glx, we found a significant postictal increase in the intersubject mean neocortical glx concentration in an animal model of prolonged generalized seizures. Assuming that differences between biochemically free and biophysically free amino acids are negligible (which may not be appropriate for glutamate, as discussed below), our glx results are in general agreement with previous findings in experimentally induced seizures in laboratory animals. Prior studies have found increases in the concentrations of glutamate, glutamine, or both, as determined by HPLC analysis of either homogenized brain samples, reflecting multiple physiological compartments (13–15), or dialysate samples, which typically reflect either intracellular or extracellular components (10–12).
Regarding published human neocortical data, one must exercise caution in comparing our postictal in vivo glx results with prior in vitro measurements of biochemically free amino acids in surgical samples obtained from patients with chronic epilepsy, particularly if glutamate and glutamine were not both measured in the same samples with the same in vitro assay. However, to the extent that spontaneously spiking human neocortex at craniotomy is analogous to the postictal state in our experiments, and assuming no decrease in glutamine (which was not measured), our results 1) are in agreement with those of Sherwin et al (9), who reported an increase in mean glutamate concentration determined by HPLC in spontaneous spiking versus nonspiking temporal lobe neocortical samples distant from, but ipsilateral to, mesial temporal epileptic foci, and 2) disagree with the results of Peeling and Sutherland (8), who found no significant difference with in vitro 300-MHz proton NMR analysis between glutamate levels in spiking versus nonspiking neocortex at the margins of hippocampal resections in patients with TLE. Peeling and Sutherland (8) also emphasized the importance of histologic condition and anatomic location of excised brain samples in reporting 1) a significant decrease in mean glutamate concentration in extracts from hippocampi with sclerotic, gliotic change versus histologically unremarkable (but electrophysiologically abnormal) hippocampi, and 2) a significant decrease in mean glutamate levels in histologically unremarkable hippocampi relative to their aggregate of spiking plus nonspiking neocortical samples.
Regarding in vitro human cortical data not specified as being from either temporal lobe neo- or archicortex, our results are in agreement with those of Perry et al (3) to the extent that human epileptic foci stimulated intraoperatively before surgical resection are analogous to the postictal state in our animal model. Perry et al (3) found an increase in total (either intracellular or extracellular), biochemically free glutamate measured with HPLC in homogenized brain samples obtained from patients undergoing neurosurgical resection of frontal (n = 2) and temporal (n = 7) lobe cortical epileptic foci confirmed by intraoperative demonstration of methohexital-induced, focally sustained EEG discharge relative to normal cortical samples obtained during removal of deep-seated brain tumors or abscesses in other patients. In a follow-up study, Perry and Hansen (4) found no significant difference in glutamine concentration between invasive EEG-confirmed, surgically removed cortical epileptic foci and control samples, while glutamate was again found to be more abundant in epileptic foci. Similarly, our results are in agreement with those of Petroff et al (6), who found that glutamate content was increased in epileptic cortex measured in surgical samples with in vitro 500-MHz analytical proton NMR (10.1 mmol/kg [mesial TLE] to 10.5 mmol/kg [neocortical TLE]) relative to in vivo 13C-MRS measurements in normal occipital cortex used as a control (8.8 mmol/kg). Epileptic cortical glutamine content was also elevated in their surgical samples (3.3 mmol/kg [mesial TLE]; 3.7 mmol/kg [neocortical TLE]) relative to control occipital cortex (4.1 mmol/kg) (6). Although not specifically stated by Petroff et al (6), it is likely that their surgical samples were resected after intraoperative demonstration of electrically or chemically stimulated, focal, sustained EEG discharges, as per modern surgical techniques in the treatment of epilepsy. Our results, and those summarized in references (3), (4), and (6), are not in agreement with earlier results of Van Gelder et al (7), who found low levels of glutamate and taurine and high glycine within the site of maximum seizure activity seen with intraoperative cortical EEG, and low γ-aminobutyric acid (GABA) and aspartic acid levels throughout the peripheral ipsilateral cortex relative to a normal range they extrapolated from literature values in humans and nonhumans.
We are aware of two published accounts of elevated postictal or ictal/periictal glx detected with in vivo proton MRS in humans. First, in a case report that prompted the current study, Fazekas et al (20) described qualitatively elevated glx at 1.5 T in the epileptogenic right parietotemporal region in a 20-year-old man after focal status epilepticus, relative to the patient's normal contralateral cerebral hemisphere. In the other, Pfund et al (30) reported their findings in 11 patients with medically intractable partial epilepsy studied with 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) and MRS. They found reduced interictal glucose metabolism and glx concentrations in (scalp and/or invasive) EEG-determined epileptic foci relative to contralateral normal brain regions in seven patients in the interictal state. Conversely, four patients examined in the ictal/periictal state had elevated glucose metabolism and glx concentrations within the seizure foci. A significant correlation between cerebral glucose utilization and glx concentration led these authors to suggest a possible coupling between glucose metabolism and the glutamate/glutamine/GABA neurotransmission cycle that is maintained in both the ictal/periictal and interictal states in epilepsy patients (which assumes that elevated glx was not fully explained by increased protein synthesis, TCA cycle activity, hyperemia, etc) (7). To the extent that the human ictal/periictal state corresponds to our postictal experiments, and that the human interictal state corresponds to our control runs, our results are in agreement with the (normalized) glx concentration results reported by Pfund et al (30).
One, or a combination, of three possible explanations may account for the demonstrated increase in the mean neocortical, postictal glx concentration in the current study. First, the net concentration of (total) biochemically free glutamate and glutamine, as detected with HPLC of tissue extracts, may increase after seizures owing to rapid hemodynamic delivery or de novo synthesis of these amino acids in situ. Second, the net (total) free glutamate and glutamine content as determined by HPLC may remain fixed, but a fraction of glutamate or glutamine that is invisible (nonbiophysically free) by MRS in control subjects may become visible after seizures. Finally, an increase in glutamine with a corresponding stoichiometric decrease in glutamate as determined by HPLC could account for the increase in the observed in vivo glx concentration, provided that the decrease of glutamate determined in vitro was derived from the portion that was invisible on MR spectra in control subjects.
In support of possible postictal changes in MRS visibility, to the extent that prolonged seizures and anoxia demonstrate similar excessive glutamate neurotransmission, 21% to 25% of cerebral glutamate measured in vitro was determined to be invisible by MRS in control subjects (normal physiological conditions) relative to anoxic animal models (31, 32). Glutamate concentrations quantified from in vivo proton MRS performed at 7.0 to 9.4 T have been found to be (up to 20% to 35%) less than those assayed with classic biochemical methods (33). Similarly, glutamate, glutamine, and alanine concentrations measured with in vitro analytical NMR have been found to be less than levels determined chromatographically (34). Noting a discrepancy between biochemical and in vivo MRS-detection assays, it has been suggested that intracellular glutamate sequestered in the neurotransmitter pool (synaptic vesicles) is the major contributor to the MRS-invisible pool (35). Thus, assuming that the postictal increase in the glx concentration that we observed was due primarily to an increase in synaptic transmission of glutamate resulting in an increase in the MRS-visible glutamate fraction, two implications are evident. First, the increase in the glx concentration measured with 0.5-T MRS is consistent with the excitatory amino acid neurotoxicity hypothesis in which excessive N-methyl-d-aspartate receptor activation triggers a cascade of toxic biochemical processes (36). Second, our results are consistent with those of other experimental studies in which extracellular glutamate was found to be increased after seizures (10–12).
On the other hand, evidence supporting an increase in glutamine rather than glutamate includes the finding that total free glutamine was increased while total free glutamate was either increased (12) or decreased (13–15) stoichiometrically after experimentally induced seizures. This stoichiometric relation, in which glutamate decreases with a corresponding increase in glutamine, would be consistent with the results presented here, provided that the decrease in glutamate occurred within an MRS-invisible (proposed neurotransmitter) pool in control subjects (35). Although the MRS visibility of glutamine has not been presented in the literature, it can reasonably be assumed that the majority of glutamine is MRS-visible, since its synthesis and degradation are co-localized with the MRS-visible mitochondrial (metabolic pool) glutamate (19).
N-Acetylaspartate
No significant postictal changes were observed in our intersubject NAA concentration estimates. This result suggests neuronal integrity with intact mitochondrial energy pathways for up to 3 hours after 30 minutes of sustained generalized seizures, since NAA is a putative marker of neuronal integrity and mitochondrial energy metabolism (37, 38). Stable postictal NAA is in agreement with in vitro analytical proton NMR results in a neonatal dog model in which no difference among control, flurothyl-induced (39) and diazepam-arrested ictal, and 1-hour postictal NAA levels were found (40). Changes in neuronal integrity and mitochondrial metabolic pathways may be delayed, occurring many hours or days after the seizure insult, and therefore not detected during our MRS experiments. Additionally, perturbations in NAA-utilizing pathways may be dependent on the seizure duration. Our 30-minute generalized seizure stimulus may poorly represent the impact of accumulated seizures in patients with chronic epilepsy, which suggests the possible utility of similar postictal experiments with more sophisticated animal models.
Creatine
In brain and smooth muscle cells, the ATP energy currency is complemented by another high-energy system involving Cr, PCr, and inorganic phosphate. Cr is synthesized by liver and kidney enzymes and transported to the brain, which maintains a tightly regulated parenchymal pool under normal physiological conditions. The ratio of PCr to Cr is governed primarily by the enzymatic activity of creatine kinase in response to cellular energy requirements (41, 42). Concurrently, the total concentration of Cr and PCr reflected in the proton MRS resonance is hypothesized to change in response to osmotic equilibrium in pathologic conditions (38).
The PCr resonance has been studied with in vivo 31P-MRS during bicuculline-induced (41, 43) and flurothyl-induced (39) status epilepticus in chemically paralyzed and oxygenated adult and neonatal animals. Ictal findings have included a decrease and plateau of PCr, corroborated by enzymatic analysis (43), a decrease and plateau of intracellular pH (pHi), and little (43) or no significant change in ATP (39) relative to baseline. The latter finding indicates no radical alterations in the energy state of the brain if adequate oxygen and glucose levels are maintained. Phosphocreatine was found to return to a level near baseline, while lactate remained elevated for a prolonged postictal period after flurothyl-induced, diazepam-reversed seizures (40).
We are not aware of any reports concerning concentration measurements of the postictal total Cr pool (PCr plus Cr) in adult animals measured with either in vivo proton MRS or in vitro enzymatic or analytic proton NMR measurements of extracted tissues. Young et al studied flurothyl-induced status epilepticus (39) and flurothyl-induced, diazepam-arrested seizures (40) in neonatal dogs with a combined in vivo proton MRS and 31P-MRS technique. Their 1331 spin-echo sequence for frequency-selective in vivo proton MRS excitation and detection was restricted to the lactate resonance band and excluded the Cr pool, thus precluding a direct comparison with the current results. However, separate in vitro analytical proton NMR (500-MHz) measurements of PCr and Cr concentrations in perchlorate-extracted neonatal tissue were performed by the same lab, which when summed would represent the total Cr pool. Young et al (39) demonstrated similar baseline and 3-hour ictal values (5.6 and 5.8 mmol/kg, respectively), and similar baseline, 1-hour ictal, and 45-minute postictal (diazepam-arrested) values (5.1, 5.3, and 5.3 mmol/kg, respectively) (40). Generally speaking, these results in neonatal dogs are not in agreement with our finding of a 23% drop in the postictal Cr pool in adult animals. Although one intuitively suspects adequate statistical power, unfortunately, a formal power analysis cannot be properly applied to the total Cr pool data tabulated in the neonatal dog studies of Young et al to determine whether their sample sizes (n = 6 controls, n = 7 flurothyl-induced, diazepam-arrested dogs) and measurement variances would have permitted detection of a 23% drop in the postictal (diazepam-arrested) Cr pool. Since PCr and Cr cannot be considered chemically or statistically independent, the variance of their sum (total Cr pool) cannot be determined without knowledge of their covariance (44).
Recently, kainic acid–induced generalized seizures have been studied by Najm et al (45) in hippocampi of isoflurane/oxygen/nitrous oxide–anesthetized rats by proton MRS at baseline, during seizures (duration not specified), and at 2-, 5-, 24-hour, and 7-day postictal time points. A significant increase in the NAA/Cr ratio was observed at the 2-hour postictal time point, which subsequently returned to baseline at 5 hours and fell below baseline at 24 hours and at 7 days after ictus. A significant elevation in the lactate/Cr ratio was observed at 2, 5, and 24 hours after ictus, which returned to baseline at 7 days. Najm et al (45) attributed changes in the ratios to changes in NAA and lactate in the numerators. In light of the current results, their observations at 2 hours after ictus might also be explained by stable NAA, decreased Cr, and increased lactate concentrations, assuming that differences in convulsants, anesthetics, animal species, and brain location (archicortex vs. neocortex) between the two sets of experiments were negligible.
Breiter et al (46) compared proton MRS–derived NAA, Cr, and lactate concentrations between scalp EEG-defined seizure foci and contralateral brain (control) in 13 patients with partial epilepsy. Ten patients were studied interictally and three were studied in a state of epilepsia partialis continua (Rasmussen syndrome) or 2 hours after ictus (acute encephalitis). Of the three patients studied during or 2 hours after ictus, two had decreased Cr.
Lactate Plus Free Fatty Acids
The physiological compartmentalization and metabolic role of brain lactate at baseline and after noxious stimuli are subjects of high current interest. Lactate is produced in the brain under normal physiological conditions (47–51) and is present in concentrations similar to that of glucose (about 1 mM), just at or below the detection threshold of MRS (38). However, under pathologic conditions, neurons and astrocytes may become more dependent on glycolytic metabolism and less dependent on oxidative metabolism to maintain intracellular ATP levels, thus accumulating lactic acid. Our qualitative observations of elevated lactate plus free fatty acids are consistent with numerous prior studies that have demonstrated elevated brain tissue lactate levels by invasive means after multiple epileptic stimuli, including electrical shock, exogenous glutamate, and chemical convulsants in animal models (15, 17, 52–54). Data from difference (subtraction) in vivo proton spectra obtained at 1.89 T between control subjects and patients after status epilepticus have shown a single peak at the same chemical shift as lactate (18).
Siesjo et al (55), using invasive microcatheter techniques in animal studies, determined that upon initiation of flurothyl-induced seizures, and coincident with spreading depression, extracellular pH (pHe) falls rapidly, a condition they attributed to changes in the Na+/H+ equilibrium. Thereafter, a prolonged decrease in pHe reflected the nonionic diffusion of lactic acid from the intracellular space to the extracellular fluid, which slowly returned to the baseline pHe value approximately 45 minutes after the cessation of seizures. Similarly, combined in vivo proton and 31P studies have demonstrated a rapid fall in pHi and a rise in lactate. Lactate and pHi become dissociated when pHi normalizes more rapidly than lactate, reflecting metabolic (thus perhaps physiological) compartmentation (42). Accordingly, recent studies have shown that within the brain, lactate can be used by neurons as an energy source during stress because there exists a lactate shuttle that operates between glial cells and neurons (56–60). Prolonged postictal brain lactate levels may have clinical application in localizing seizure foci with postictal MRS (61).
As for our qualitative observations, time series plots of quantitative brain and blood lactate (and/or arterial blood gas pH) levels during prolonged seizures revealed a close relationship during the initial phase of seizure activity. However, this close temporal relationship between systemically and cerebrally produced lactate has not been studied extensively (40). Increased arterial lactate during prolonged seizures may arise from a primary myocardial or skeletal muscle lactic acidosis associated with a seizure-induced increase in serum catecholamines (39).
Regarding free fatty acids, it is unlikely that our qualitative postictal results reflect contamination from marrow or scalp fat exacerbated by head motion, because 1) lactate and free fatty acids were not qualitatively observed in control spectra and 2) animals were chemically paralyzed to prevent head motion (Fig 1). A broad-band 1.5-T MRS signal observed repeatedly at 1.2 ppm in humans after electroconvulsive therapy (62), and demonstrated in the present study (Fig 3), is characteristic of the methylene protons of long-chain fatty acids (63). Furthermore, the liberation of free fatty acids in MRS-visible quantities from excitatory amino acid–mediated processes has also been observed in experimental animal models (64, 65).
Taken together with the increase in postictal glx concentration, our qualitative findings of elevated and lactate plus free fatty acid levels are consistent with the findings of Magistretti et al (52) and Pellerin et al (54), in which extracellular glutamate was shown to stimulate glycolytic lactate production. The separation of lactate from free fatty acid resonances with long-TE spectra, which was beyond the scope of the present study, would be informative in future work.
Study Critiques and Limitations
This study used a cerebral water-referenced quantification algorithm. The algorithm is dependent on accurate knowledge of tissue water content and metabolite relaxation times. Prior studies of brain water content measured in excised samples by analytical desiccation (reference standard) or microgravimetry have reported an increase of up to 11 percentage points within the entire cerebrum of experimental animal models of hyponatremia (66), an increase of 9 percentage points within human autopsy samples located adjacent to hematomas and neoplasms (67), and an increase of about 10 percentage points in human autopsy samples obtained from both peritumoral white matter and cortex (68, 69). Therefore, any brain edema associated with PTZ-induced seizures in our study could have introduced a positive bias in each of the metabolite concentration estimates, since the estimates are proportional to the water content in Equation 2 (26). Although T2-weighted MR images obtained between 2 and 3 hours after ictus were unremarkable, the possibility of edema detectable with fluid-attenuated inversion-recovery (FLAIR) or diffusion-weighted (DW) imaging apparent diffusion coefficient maps (70, 71) could not be excluded. Unfortunately, neither FLAIR nor DW sequences were available with our equipment.
The relaxation times used in control and postictal calculations were measured in healthy adult human volunteers. However, under pathologic conditions, slight changes in cerebral water content may result in large variations in T1, as the two quantities are linearly related over a narrow range (72). This relation has been demonstrated previously in cancerous animal tissues (73–75) and in human brain specimens (69, 76). Under the current experimental protocol, it was impractical to measure either longitudinal or transverse relaxation times during both control and stimulus experiments owing to time constraints. Relaxation time measurements and estimates of cerebral water content based on spin-density MR imaging (26, 77, 78) obtained under pathologic conditions may prove beneficial to future work.
Generalized seizures as monitored by scalp EEG were induced in chemically paralyzed adult mongrel dogs by repeated intravenous injections of PTZ, a known GABA antagonist (79). The choice of a GABA antagonist for this animal model was validated by the clinical use of antiepileptic agents known to increase GABAergic transmission or alter GABA metabolism, such as vigabatrin and valproic acid (80). However, potential differences in the mechanism of seizure production, in seizure duration, and in the MRS voxel location studied in the animal model and in patients with chronic seizure foci could limit the clinical applicability of this study. Furthermore, it is possible that postictal metabolite levels may be PTZ dose-dependent, and thus contribute to variability observed across subjects. Other variables, such as the sex and age of the animal, as well as any unknown medical condition, were not controlled and may have contributed to the biological variability observed with MRS.
The time required to check and manually adjust shimming and water-suppression parameters when needed before the acquisition of each reference water and metabolite spectrum was nonuniform in our experiments. Therefore, metabolite fluctuations in control versus postictal spectra as a function of time could not be reliably analyzed with the repeated-measures ANOVA method. However, consideration of physiological and biochemical responses as a function of time may prove beneficial in future studies.
The use of MRS at 0.5 T rather than 1.5 T in this study was based on magnet time availability at our institution and on a study by Prost et al (24), in which the signal-to-noise ratio (SNR) of glx was improved at 0.5 T relative to 1.5 T by a factor of 2. This improvement was attributed to 1) a decreased spectral linewidth resulting from improved main-field homogeneity (expressed in units of Hz) as compared with higher-field-strength magnets with comparable main-field homogeneity (expressed in units of ppm), 2) decreased overall T1 values, which improve SNR per unit time, and 3) a collapse in the γ and β multiplet resonances into a pseudosinglet that allows for a cerebral water-referenced, lorentzian-fitted area quantification algorithm.
Unlike glx, the SNRs of Cr and NAA at 0.5 T are decreased relative to values observed at 1.5 T (24). Therefore, statistical variations (measurement errors) (81) in the calculated Cr and NAA concentrations in control and postictal experiments would be expected to be greater at 0.5 T than at 1.5 T. Another possible source of measurement error for Cr, more so than for glx or NAA, may be its closer proximity to the CHESS water-suppression pulse, regardless of main magnetic field strength. Given the measurement variances with our system for control and postictal runs (see Table), a greater number of animals with statistically significant intrasubject mean changes in glx, Cr, and perhaps NAA would have required either that more than approximately a dozen samples be taken in each 3-hour block of available magnet time or that longer experiments be conducted, both of which were impractical.
Summary
We have demonstrated significant changes in the intersubject mean glx and Cr concentrations obtained with in vivo proton MRS at 0.5 T, and no change in NAA within a 3-hour postictal period. The quantitative glx and NAA results presented are in general agreement with those of other studies reported in the literature. The use of the cerebral water-referenced concentration estimate algorithm bridges the gap between MR spectra interpreted by visual inspection and accepted metabolite concentrations measured by HPLC and other classical biochemical assays. Direct correlation of MRS-derived concentration estimates in postictal animal models of TLE with concentrations obtained using microdialysis catheter recordings or biochemical assays of excised brain samples in the same subjects would be a desirable extension of this study. Additionally, the future use of 13C-MRS, ultra-high-field-strength in vivo proton MRS, or the application of DW (82), deconvolution (83), or correlation spectroscopy techniques to in vivo proton MRS may resolve the relative contributions to, and the physiological compartments of, glutamate and glutamine within the glx peak in the proton spectrum.
Footnotes
1 Address reprint requests to Scott D. Rand, MD, Department of Radiology, Medical College of Wisconsin, Froedtert Memorial Lutheran Hospital, 9200 W Wisconsin Ave, Milwaukee, WI 53226.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.
- 49.
- 50.
- 51.
- 52.↵
- 54.↵
- 55.↵
- 56.↵
- 57.
- 58.
- 59.
- 60.
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.
- 72.↵
- 73.↵
- 74.
- 75.
- 76.
- 77.
- 78.
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- Received August 3, 2000.
- Accepted after revision June 22, 2001.
- Copyright © American Society of Neuroradiology