Abstract
Summary: Intrathyroidal lymphoepithelial cysts are rare, and only 15 such cases have been reported. Although sonography has been performed in some cases, the findings have not been discussed previously. Despite its rarity, the sonographic appearances of this lesion are similar to those of other commonly encountered congenital cystic lesions in the head and neck, such as thyroglossal duct cysts and second branchial cleft cysts, and this may provide a clue to its diagnosis. We describe the sonographic appearances of intrathyroidal lymphoepithelial cysts.
Branchial cleft cysts are common in the lateral neck; however, cysts with histologic features of branchial cysts have been reported in unusual locations, such as the thymus, oral cavity (1), parotid gland (2), pancreas (3, 4), and thyroid (5–11). Since the first report of intrathyroidal lymphoepithelial cyst (ILCs) in 1989 (9), only 15 cases of ILCs have been reported in the literature. We present another case of ILCs and discuss the sonographic appearances that may provide a clue to the diagnosis of this rare condition.
Case Report
A 64-year-old man presented with a 4-month history of a palpable thyroid mass. There was no associated pain, hoarseness of voice, or obstructive symptoms, and the results of his thyroid function tests were normal.
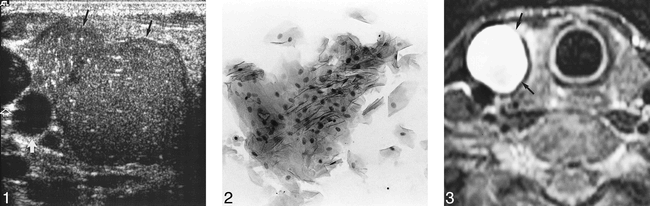
High-resolution (7.5–10 MHz) sonography (ATL HDI 5000; Bothell, WA) of the thyroid revealed a 5-cm, loculated, well-defined, noncalcified nodule occupying most of the right lobe of the thyroid. It showed diffuse, heterogenous, internal echoes, with multiple, tiny echogenic foci exhibiting a comet tail artifact (Fig 1). There was no posterior enhancement, and the lesion was avascular on power Doppler sonograms. However, when pressure was applied on the mass with the transducer, the entire contents shifted, suggesting its cystic nature. A small, 6-mm, well-defined cystic nodule was seen in the left lobe, which was otherwise normal.
Transverse sonogram of thyroid showing multiloculated nodule (black arrows). Note homogeneous internal architecture and presence of comet tail artifact within (small white arrows) created by cholesterol crystals. This represents the pseudosolid appearance of a congenital neck cyst. Transducer pressure on nodule produces shifting of contents, suggesting its cystic nature. Aspiration yielded viscous yellowish puslike fluid. Open arrow identifies internal jugular vein, and large white arrow marks common carotid artery.fig 2. Clump of mature squamous cells (hematoxylin and eosin stain; original magnification, ×200).fig 3. T2-weighted (2500/100 [TR/TE]; number of acquisitions, two) axial-view image obtained through thyroid shows well-defined, markedly hyperintense cystic nodule (arrows) in right lobe of thyroid. There are no other specific features to suggest that it may be of congenital origin
A sonographically guided fine-needle aspiration yielded 10 mL of yellowish puslike material. The nodule diminished in size after the aspiration.
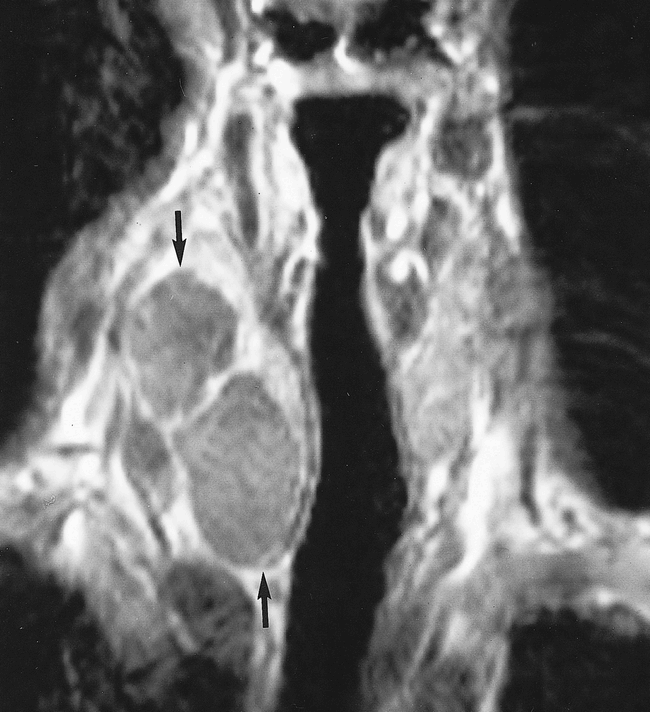
On cytologic smears, there were many mature superficial squamous cells with intact nuclei, anucleate squames, clusters of neutrophils and lymphocytes, macrophages, and in the background, amorphous debris (Fig 2). The findings were compatible with a branchial cyst, but a well-differentiated squamous cell carcinoma or a thyroglossal duct cyst were also possibilities. In view of the possibility of a squamous cell carcinoma metastatic to the thyroid, MR imaging of the neck was performed and revealed a 22-mm, cystic nodule in the right lobe of the thyroid, markedly hyperintense on T2-weighted sequences (Fig 3) and slightly hyperintense on T1-weighted sequences (Fig 4). The mass was completely intrathyroidal with no extrathyroidal extension. No other mass lesion was detected in the neck.
T1-weighted (500/20; number of acquisitions, one) coronal-view image shows multiloculated, slightly hyperintense nodule (arrows) in right lobe of the thyroid
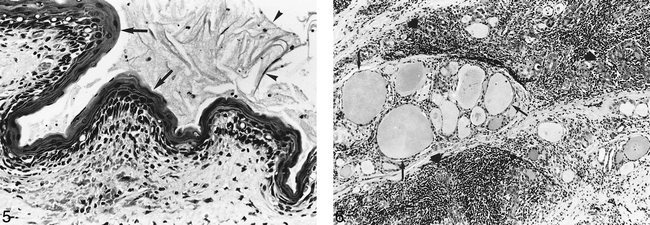
A right hemithyroidectomy was subsequently performed. Macroscopically, a cyst with two loculi, 2 and 2.5 cm in diameter, respectively, was seen in the right lobe of the thyroid. It contained thick yellow-tan–colored material and had a granular lining. Microscopically, the cyst was lined by squamous epithelium with evidence of keratin formation (Fig 5). Keratin, anucleate squames, and cell debris were present in the cyst. In some places, the epithelium was absent and granulation tissue with cholesterol crystal clefts and multinucleated giant cells were found. No respiratory epithelium was identified. Supporting the epithelium was a zone of dense collagen, and beyond this were colloid-containing thyroid follicles of variable size. A striking feature was dense lymphocyte infiltration of the thyroid tissue (Fig 6). Clear-cut germinal centers were not identified. The histologic findings were those of an intrathyroid ultimobranchial cyst with chronic lymphocytic thyroiditis.
Higher-power view shows squamous epithelium (arrows) and desquamating keratin (arrowheads). Lymphocytes are seen in the underlying connective tissue (hematoxylin and eosin stain; original magnification, ×250).fig 6. Thyroid follicles of variable size (arrows) can be seen in this view. Some are compressed by diffuse lymphocyte infiltrate (large arrows) (hematoxylin and eosin stain; original magnification, ×100)
Discussion
The pathogenesis and embryologic basis of ILC is uncertain (5–7, 9, 11), and a detailed description is beyond the scope of this report. The presence of squamous epithelium (as seen within the wall of the cysts in question) is unusual within the thyroid gland. It is therefore essential to consider the source of squamous cells within the thyroid and the probable origin of ILC. Squamous epithelium in the thyroid may be derived from five sources (12): thymic remnants, thyroglossal duct remnants, metaplastic follicular cells, ultimobranchial remnants, and tumors containing squamous cells.
Thymic remnants have been identified in the lateral parts of the thyroid gland (12). They likely occur because, embryologically, the thymus has a close relationship with the thyroid (13). No evidence of thymic tissue was seen in our case, and it has been described in only one of the six previous reports (5–11). Therefore, it is unlikely that the cyst in our case is of thymic origin. Thyroglossal duct remnants can also be a source of squamous cells in the thyroid (12), but they are centrally located rather than in the lateral lobes.
Squamous cells may also be found in thyroid glands affected by inflammation (14) and probably originate from remnants of the ultimobranchial body (also known as solid cell nests) rather than metaplastic follicular cells (14). Cysts lined by squamous epithelium have been described in the adult, vitamin A–deficient rat (15). There are no such reports regarding humans, and the patient in this study had a normal diet and no obvious evidence of vitamin A deficiency.
A range of cystlike lesions is seen in tumors containing malignant squamous epithelium (adenocanthoma, adenosquamous carcinoma, or pure squamous carcinoma). Because many of these appear to arise from follicular epithelium, it is most likely that squamous metaplasia that has undergone malignant degeneration has taken place in these neoplasms (12). There are also reports of association of ILC with papillary carcinoma of the thyroid (5, 10). In our case, however, there was no evidence of malignancy.
Most authors link the origin of the ILC to the remnants of the ultimobranchial body (5, 7, 10, 13), also known as solid cell nests (16–18). The ultimobranchial bodies in the human embryo develop from the fourth and fifth branchial pouch complexes along with the thymus and parathyroid tissue. They later become incorporated within the lateral lobes of the thyroid as tiny cystic and solid cell nests and are thought to be involved in the development of parafollicular cells (19, 20). Although these benign developmental rests are common in all age groups and were recognized by Getzowa in 1907 (21) as ultimobranchial body remnants, they often are not included in the differential diagnosis of thyroid lesions. Because the ultimobranchial body remnants are very small, their reported incidence depends on the extent of tissue sectioning and varies accordingly in literature (22). In autopsy series, the incidence rate in adults was 60% (16).
The formation of branchial-cleft-like cysts in the thyroid is strongly associated with Hashimoto's thyroiditis (9). It, however, is not an absolute prerequisite (6, 7, 11). The presence of lymphocytic infiltration adjacent to the cyst is a consistent finding, raising the possibility that ILC can arise whenever there is inflammation of any type (11). The curious relationship of these cysts to Hashimoto's thyroiditis echoes the clinical observation that branchial cleft cysts enlarge and frequently present after an upper respiratory tract or dental infection (23). One may therefore speculate that the thyroiditis may induce enlargement of previously existing ILC.
Is it possible to suggest the diagnosis of ILC preoperatively? In view of the rarity of the lesion, the diagnosis can be suggested only postoperatively, as was the case in all the previous case reports (5–11). In routine clinical practice, the most common cause for a patient presenting with a painless enlarging mass in the thyroid is hemorrhage within a thyroid nodule in a multinodular goiter (8, 24), and sonography is often the initial imaging investigation. Sonographically, hemorrhage within a thyroid nodule is seen as a complex cystic mass with internal septations, debris, thick walls, and, in the acute phase, a fluid/debris level. Aspiration of such a mass usually yields altered blood that is dark brown in color. The presence of a comet tail artifact in a cystic thyroid nodule has also been previously described (25) and is thought to be due to reverberations caused by inspissated colloid.
We reviewed the previous case reports to evaluate the sonographic appearances described for ILC (none of the previous cases included MR imaging examinations). Sonography was performed in three of the cases previously reported (6, 8, 11). Based on sonographic findings, Carney (6) described the cyst as a fluid-filled cavity of spongy consistency containing a large amount of suspended debris. Ryska et al (11) called it a solitary cystic nodule, and Lim-Tio et al (8) described it as a heterogeneous echogenic nodule with a hypoechoic rim and an aspiration yielding creamy green fluid. In our case, the cyst was uniformly echogenic with suspended debris within and showing the presence of a comet tail artifact. Thus, in two of the four cases, the cyst was echogenic, showing suspended debris in one and being “cystic” only in one. Therefore, although it is developmentally a cyst, it does not exhibit the typical sonographic appearances of a cyst, such as anechoic lesion with thin walls and posterior enhancement. This is not surprising considering that congenital cystic lesions of the neck, such as second branchial cleft cysts (12%) (26) and thyroglossal duct cysts (27.5%) (27), often show a pseudosolid appearance because of the presence of cellular material, cholesterol crystals, and keratin within the cyst (28). The uniform echogenicity may lead to an erroneous assumption that the lesion is solid, especially if posterior enhancement is absent. When pressure was applied on the cyst with the transducer, however, the entire contents shifted, suggesting its cystic nature. The comet tail artifact within the cyst in our case was probably due to the presence of cholesterol within the cyst, as confirmed by histologic analysis. Such an artifact is frequently seen in the gallbladder, and it is well established that it is due to reverberation caused by cholesterol crystals (29). Thus, the sonographic appearances of an ILC seem to be similar to other congenital cystic lesions in the neck rather than hemorrhage within a thyroid nodule in multinodular goiter.
Conclusion
Although the diagnosis of ILC will invariably be made after surgery, fine-needle aspiration of yellowish, green viscous fluid (rather than altered blood) and the sonographic appearances of a pseudosolid, cystic nodule in the thyroid should alert the sonographer to the possibility of a congenital developmental cyst rather than the more common hemorrhage within a thyroid nodule.
Footnotes
↵1 Address reprint requests to Anil T. Ahuja, FRCR, Department of Diagnostic Radiology and Organ Imaging, Prince of Wales Hospital, Shatin, N.T., Hong Kong.
References
- Received October 25, 1999.
- Accepted after revision February 15, 2000.
- Copyright © American Society of Neuroradiology