Abstract
BACKGROUND AND PURPOSE: Factors predictive of primary brain tumor outcome have been studied extensively, although the prognostic value of radiologic data, such as MR imaging and angiographic characteristics, has not been studied in depth. The purpose of this study was to determine whether radiologic data were prognostic factors among patients with recurrent glioblastoma multiforme and anaplastic astrocytoma treated with selective intra-arterial chemotherapy.
METHODS: Forty-six patients were enrolled in a Phase II study of intra-arterial chemotherapy with carboplatin and Cereport (Alkermes Inc.; Cambridge, MA), a bradykinin analog that selectively increases permeability of the blood-tumor barrier. MR imaging volumes of enhancing tumor, resection cavity, and T2 signal abnormality were measured with T1-weighted and T2-weighted sequences. Volumes were analyzed individually and in various combinations. Tumor vascularity was graded on angiograms. Outcome was measured by time to tumor progression and survival.
RESULTS: Of 46 patients included in this study, 41 underwent evaluation. Thirty were male and 11 were female; mean age was 48.5 years. Karnofsky scores ranged from 70 to 100. Thirty-two patients had glioblastoma multiforme, whereas nine had anaplastic astrocytoma. Twenty-eight patients had tumor progression and 13 had stable disease. Twenty-three patients died after an average of 205 days; 18 were surviving at an average of 324 days from the start of intra-arterial chemotherapy. In multivariate analysis, time from diagnosis to intra-arterial chemotherapy was predictive both of time to tumor progression and survival. Net tumor volume and vascularity also were significant for survival. Age, Karnofsky performance status, histologic findings, gender, MR imaging area, resection cavity volume, T2 signal abnormality volume, and various combined volumes were not significant.
CONCLUSION: If confirmed by further studies, radiologic factors such as tumor volume and angiographic vascularity should be considered in design and stratification of future chemotherapy trials.
Glioblastoma multiforme and anaplastic astrocytoma are the most common primary brain tumors occuring during adulthood (1). Incidence of these tumors has been increasing since the 1950s (2–4). Standard treatment is surgical resection followed by radiation therapy (5), but prognosis is dismal. This is partly because of the high likelihood of recurrence, which usually is within 1 year of initial treatment (6). The benefit of adding intravenous chemotherapy to the treatment regimen has been very modest (7). Intra-arterial chemotherapy is an experimental mode of administration with the main advantage of achieving a higher drug-tissue level compared with intravenous delivery, while minimizing systemic drug toxicity (8). Clinical trials of intra-arterial chemotherapy, however, have had modest and variable efficacy. The favorable responses of some patients to intracarotid delivery of cisplatin and carboplatin indicate that certain patients, for unknown reasons, may benefit more from this technique than others (9, 10).
Evaluation of prognostic variables is essential to ongoing clinical trials involving primary brain tumors. Patients likely to benefit from a given treatment can be included, while those who may do worse can avoid related toxicities. Identification of patients with similar prognoses allows selection of a more homogeneous experimental population and improved randomization. Therefore, statistically significant results could be drawn from smaller, less elaborate trials, limiting exposure to possible toxicity and cost.
Factors predictive of prognosis of primary brain tumors have been extensively studied. Age, Karnofsky performance status, and histologic characteristics of tumor are the most accepted prognostic features (11–17). The prognostic value of radiographic data, such as MR imaging and angiographic characteristics, has not been examined at length.
Our purpose was to define and evaluate the combined prognostic value of MR imaging characteristics, angiographic findings, and clinical information. We retrospectively reviewed the clinical and radiologic data of patients with recurrent primary brain tumors enrolled in a Phase II study of intra-arterial chemotherapy with carboplatin and Cereport (Alkermes Inc.; Cambridge, MA), a blood tumor–barrier modifier. Certain radiologic factors—small enhancing tumor volume on T1-weighted MR imaging scans and absence of tumor hypervascularity on angiograms—were significant for survival. These results, if confirmed by further studies, could help to improve patient selection and stratification for future intra-arterial chemotherapy trials.
Methods
We retrospectively reviewed the clinical and radiologic data of 46 patients enrolled in an open-label, single-arm, Phase II study of the intra-arterial administration of carboplatin and Cereport for the treatment of recurrent glioma. Patients were enrolled in the study upon radiographically measurable recurrence of a pathologically documented glioma. Detailed inclusion and exclusion criteria are listed in Table 1. All patients were treated with intra-arterial carboplatin (600 mg/hemisphere) and Cereport (300 ng/kg) at our institution and six other centers between January 1994 and July 1997. Cereport is a bradykinin analog that temporarily increases the vascular permeability of the blood-tumor barrier. This effect has been confirmed experimentally and clinically (18). Intra-arterial delivery was performed by selective catheterization of the internal carotid artery above the ophthalmic artery in the anterior circulation and of the posterior cerebral or basilar artery in the posterior circulation. Doses were based on regional cerebral blood flow. The dose calculation method assumes each vascular territory receives its fractional arterial supply in the following proportion: middle cerebral artery 60%, anterior cerebral artery 20%, posterior cerebral artery 15%, and perforating arteries 5%. A detailed explanation of the dose calculation method based on cerebral blood flow has been previously discussed (19).
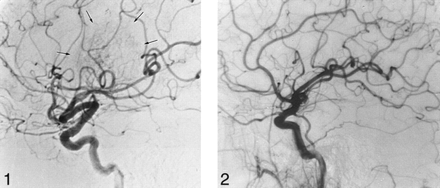
Subject selection criteria
Clinical variables that were analyzed included age, sex, and days from diagnosis to initiation of intra-arterial chemotherapy. Radiographic variables were evaluated on baseline studies by a reader blinded to outcome and included tumor vascularity, MR imaging enhancing tumor area, and MR imaging tumor-related volumes. Tumor vascularity was graded on pretreatment angiograms as 0, avascular; 1, mild blush; 2, moderate blush with thin network of abnormal vessels; 3, diffuse opacity with polymorphic vessels or flakes; and 4, arteriovenous shunting (Fig 1 and 2). Angiograms were interpreted in conjunction with corresponding MR imaging scans to ensure that large resection cavities (where lack of tissue would create the appearance of avascularity) were not mistakenly graded as avascular lesions. MR imaging area was measured as the cross product of perpendicular slice dimensions on the slice depicting the largest transverse area of the tumor. MR imaging volumes were obtained from digitized scans using a computerized program (Evergreen Technologies Inc, Castine, ME) allowing more accurate volume measurements than the commonly used cross-product method, which assumes every volume to be spherical or ellipsoid (20). Regions of interest were manually traced on each digitized slice (Fig 3 and 4), and the sum of slice volumes gave a total volume for each variable. Measured MR imaging variables were as follows: 1) contrast enhancing tumor, with cystic and resection cavities included; 2) resection cavity (potentially including tumor necrosis and cystic fluid) on either gadolinium-enhanced T1-weighted scans or T2-weighted scans, whichever better demonstrated the cavity; and 3) T2 signal abnormalities (potentially including nonenhancing tumor, edema, and radiation-induced changes), with cystic and resection cavities and enhancing tumor included. Other volumes calculated from these measured values were: 1) net edema volume (which excluded enhancing tumor and resection cavity); 2) combined net edema and net enhancing tumor volume; 3) combined net resection cavity and net edema; and 4) net contrast-enhancing tumor.
Hypervascular tumor. Right carotid artery angiogram shows displacement of the branches of the middle cerebral artery. The tumor blush is indicated by the arrows.fig 2. Hypovascular tumor. Right carotid artery angiogram shows no tumor blush. The mass effect exerted by the tumor causes displacement of the insular branches of the middle cerebral artery as they enter the Sylvian triangle
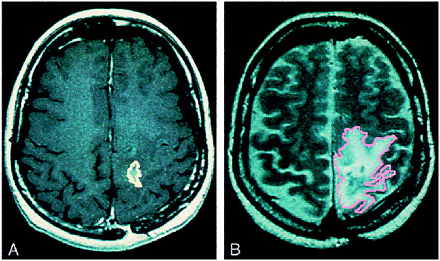
These MR scans show regions of interest manually traced on digitized scans to obtain volume measurements. Enhancing tumor is outlined in yellow and T2 signal abnormalities are outlined in pink. A, Contrast-enhanced T1-weighted sequence shows a small enhancing tumor in the left anterior parietal area. B, T2-weighted sequence shows a large and irregularly shaped hyperintensity representing tumor edema
Outcome measures included the following continuous variables: 1) tumor progression based on the earliest occurrence of frank clinical deterioration, increase in MR imaging area (at least 25% increase from baseline) or MR imaging volume (at least 50% increase from baseline); and 2) days of survival from initiation of intra-arterial chemotherapy.
Data were analyzed using both univariate analysis and the Cox proportional hazards regression model; death rates and disease-progression rates were assumed to be modeled as log-linear functions of the covariates to quantify the relationship between tumor progression or survival and our set of clinical and radiologic variables. Prognostic factors were selected as candidate factors for multivariate analysis when the independent P value after each stepwise addition of variables was less than .10. The multivariate Cox's proportional hazards model was run first with survival, and then with time to tumor progression, as the outcome variable.
Results
Of 46 patients included in the study, five withdrew before the second cycle of chemotherapy; their outcome data were excluded from analysis. Forty-one patients underwent evaluation. Thirty patients were male and 11 were female. Ages ranged from 24 to 72; mean age was 48.5 years. Mean time from diagnosis to initiation of intra-arterial chemotherapy was 722 days. In most patients (n = 37), the initial diagnosis was either glioblastoma multiforme or anaplastic astrocytoma. Four patients initially presented with low-grade gliomas that later recurred with a histopathologic diagnosis of anaplastic astrocytoma. Final diagnosis was glioblastoma multiforme in 32 patients and anaplastic astrocytoma in nine.
All patients had received surgical intervention ranging from biopsy only to gross total resection. Twenty patients (49%) only had one surgery with an initial histopathologic diagnosis of glioblastoma multiforme or anaplastic astrocytoma. The remaining 21 patients (51%) had undergone multiple surgeries. The most recent surgeries performed were: 1) biopsy alone in 13 patients (32%) an average of 172 days (ranging from 21 to 456 days) before initiating intra-arterial chemotherapy; 2) subtotal resection in 15 patients (37%) an average of 184 days (ranging from 27 to 449 days) before initiating intra-arterial chemotherapy; and 3) gross total resection in 13 patients (32%) an average of 315 days (ranging from 26 to 1663 days) before initiating intra-arterial chemotherapy. Nine patients (22%) underwent surgery fewer than 60 days prior to initiating intra-arterial chemotherapy. Seven of these patients (17%) underwent subtotal or gross resections, and two (5%) had biopsies only.
Patients received two to eight monthly cycles of intra-arterial chemotherapy. The average number of cycles was four. In total, 160 infusions were performed. The most common infusion was 600 mg carboplatin into the internal carotid artery (n = 97). Other arteries also were infused with 600 mg carboplatin: middle cerebral artery (n = 12), posterior cerebral artery (n = 7), anterior cerebral artery (n = 2), and other (n = 1). Infusions were divided between two arteries if the area involved by tumor received its blood supply from two vascular territories: internal carotid artery and posterior cerebral artery (n = 32); anterior cerebral artery and middle cerebral artery (n = 5); and posterior cerebral artery and middle cerebral artery (n = 1).
Nineteen patients had avascular tumors, whereas 20 had hypervascular tumors. In two cases, reliable angiographic data were not available. Of the 20 hypervascular tumors, 12 were graded as a 1 (mild blush) and two of these patients' angiograms revealed a late venous blush. Six tumors were graded as a 2 (moderate blush) with thin network of abnormal vessels, and two were graded 3 (diffuse opacity with polymorphic vessels or flakes). No tumors were graded as a 4 (arteriovenous shunting).
MR imaging areas ranged from 540 to 6368 cm2, with a mean of 1644 cm2. Mean MR imaging volumes are stated in Table 2.
Mean values and standard deviations of MR volumes measured
Of 41 patients, 28 had tumor progression and 13 had stable disease. The average time to tumor progression was 87.6 days. At an average follow-up of 258 days, 23 patients had died after an average of 205 days, whereas 18 were surviving at an average of 324 days from the start of intra-arterial chemotherapy. Of the 23 patients who died, four had stable disease and 19 had tumor progression. In contrast, nine of the 18 surviving patients had stable disease and the remaining nine had tumor progression. Time to tumor progression was highly related to survival (P = .001).
Analysis of the number of days from initial diagnosis to recurrence showed that a large majority of tumors recurred in fewer than 2 years (n = 32, 78.0%). The remaining time to recurrence ranged from 2 to 10 years (n = 9, 22.0%). Median time to recurrence ranged from 99 to 190 days (n = 10, 24.4%).
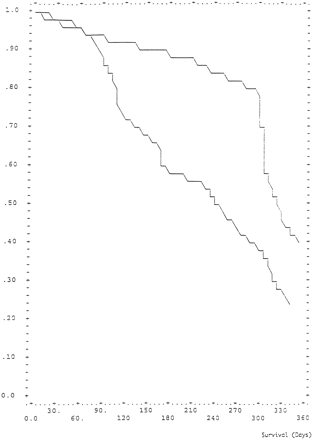
Increased number of days from initial diagnosis to recurrence predicted survival at a high significance level (P = .001) and tumor progression at a borderline significance level (P = .061). In univariate analysis, avascularity (P = .0123), and small net enhancing tumor volume (P = .01) on T1-weighted MR images were significant in predicting prolonged survival. In univariate analysis, pathologic findings and vascularity were found to be correlated. In multivariate analysis, the same three variables remained predictive of survival: 1) days from initial diagnosis to recurrence (P = .001), 2) avascularity (P = .001, Kaplan-Meier survival curve shown in Fig 5), and 3) net enhancing tumor volume on T1-weighted MR images (P = .003). Variables that were not predictive of outcome in this study included age, gender, MR imaging area and volumes of resection cavity, T2 signal abnormalities, net edema, combined net edema and net enhancing tumor, combined net resection cavity and net edema, and combined net enhancing tumor and net resection cavity.
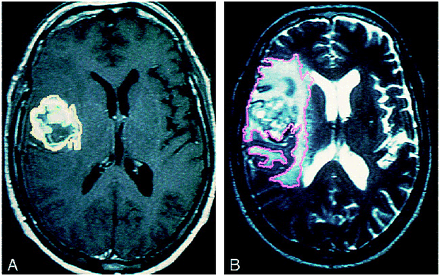
These MR imaging scans show regions of interest manually traced on digitized scans to obtain volume measurements. Enhancing tumor is outlined in yellow; resection cavity, central necrosis, and cystic fluid are outlined together in green; and T2 signal abnormalities are outlined in pink. A, Contrast-enhanced T1-weighted sequence shows a rounded, enhancing, tumor volume with central necrosis in the right insular region. B, T2-weighted sequence shows a large hyperintensity representing total tumor, resection cavity, and edema volume. Note the very irregular shape that is neither spherical nor ellipsoid. The computerized volume-rendering program better approximates volume than the commonly used cross-product method, which assumes the shape of the volume is spherical or ellipsoid
The estimated survival function using the Cox proportional hazards model and the mean value covariate pattern (days to recurrence, net tumor volume) for hypervascular and hypovascular tumors shows survival in 39 patients (those whose angiographic data were available) with recurrent primary brain tumors treated with selective intra-arterial chemotherapy. The y-axis shows percentage of patients surviving. The x-axis shows survival in days. Patients with angiographic findings of hypovascularity are represented by the upper line. Those with angiographic findings of vascularity are represented by the lower line
Discussion
This study found that radiologic factors, such as small enhancing tumor volume on T1-weighted MR images and absence of tumoral hypervascularity on angiograms, were independent prognostic factors of prolonged survival in a Phase II trial of intra-arterial chemotherapy.
One must realize that this study is biased because of the patient population, which does not represent the typical population of patients with glioblastoma multiforme and anaplastic astrocytoma because of patient recruitment and selection (Table 1). Previously described by Kirby, the problem of biased outcomes owing to patient selection is inherent to trials of intra-arterial chemotherapy and other aggressive treatment regimens. Patients who are judged eligible for experimental treatments may be the very patients who would have had a better prognosis even with standard treatment (21). Therefore, our findings cannot be applied to all malignant glioma patients, but do merit further investigation. If confirmed by additional studies in broader populations, such results may influence determination of prognostic factors in clinical trials. Evaluation of prognostic factors is vital to improving research pursuing new therapies for primary brain tumors. Patients who will benefit more from a given treatment can be identified, whereas those who tend to do worse can avoid treatment-associated toxicities and complications. Also, a more homogeneous patient population can be selected, and better randomization or stratification into various treatment arms can be achieved. Therefore, fewer patients must be enrolled to reach statistically significant results, thereby limiting cost and expediting results for accelerated clinical application.
Recognizing the necessity of established prognostic factors, many investigators have studied a wide range of variables. Those readily accepted by most investigators are age, Karnofsky performance score, and histopathologic features (11–17).
Previous studies have defined a multitude of additional significant variables for tumor response, tumor progression, and survival: 1) patient-related information, such as symptom duration (15), extent of symptoms (15), mental changes (22), focal neurologic deficits (22), seizures (16), speech impairment (11), visual disturbances (11), cranial nerve involvement (23), blood group, pretreatment white cell and platelet counts (24), World Health Organization status (22), and initial corticosteroid dependency (22); and 2) tumor-related factors, such as location of tumor (23), necrosis in the tumor (25, 26), tumor grade (27), and tumor location (28); and 3) treatment-related aspects, such as radiation dose (15, 27) and extent of surgical removal (11, 16, 23).
One challenge in evaluating treatment of primary brain tumors is defining an appropriate outcome measure. Some investigators use a combination of factors, such as neurologic symptoms, amount of steroid use, and radiographic appearance (29), but data presented can be subject to error of assessment between different investigators. These discrepancies should be taken into account when interpreting results (14). Other investigators limit subjectivity by measuring outcome as a percentage change in radiographic appearance. Ultimately, survival is the most objective and reliable endpoint (14). In this study, we measured outcome by both survival and time to tumor progression.
Three established prognostic factors—age, Karnofsky score, and histopathologic features—were not significant or not analyzed in our study of a highly selected patient population. Age is well accepted as a prognostic factor for survival of patients with glioblastoma multiforme and anaplastic astrocytoma. Nevertheless, in patient populations with more narrowly distributed age range and younger mean age, the predictive value of age may be lost. Populations biased by younger patients have been described in numerous series of intra-arterial chemotherapy (12, 13, 17, 22), probably because such patients are more appropriate candidates for an experimental and invasive treatment option. In our population, age was not predictive of tumor progression or survival. The age distribution consisted of mostly younger patients, with a mean of 48 years; 27 (66%) of 41 patients were younger than 60 years. Karnofsky score was not analyzed in this highly selected group because only patients with a score higher than 70 were included in the study. This is the standard practice for patients treated aggressively, as with intra-arterial chemotherapy, and results may be biased (21). Histopathologic findings in this patient population were mostly glioblastoma multiforme (n = 32, 78%), and a minority had anaplastic astrocytoma (n = 9, 22%). In univariate analysis, pathologic features and vascularity correlated, which was an expected result because the grade of a glioblastoma multiforme relies in part on vascularity. Therefore, vascularity instead of histopathologic grade was included in the Cox proportional hazards model analysis to assess the value of pretreatment angiograms in predicting outcome.
A long delay from initial diagnosis to recurrence was found to be significant for survival in this study. This indicates that even among tumors that are pathologically identified as glioblastoma multiforme or anaplastic astrocytoma, biologic activity varies. Tumors that recur more slowly may have biologic characteristics more similar to low-grade lesions; moreover, fast regrowth may signal progression toward malignancy (30). In previous studies, malignant gliomas with a longer time to recurrence have been associated with an increased survival (15, 31). Our study confirmed these results; in addition, increased time to recurrence also was predictive of slower tumor progression after intra-arterial chemotherapy.
Is tumor volume associated with survival? In considering the prognostic significance of tumor volume, it is important to note that glioblastoma multiforme and anaplastic astrocytoma have a characteristic tendency to infiltrate surrounding brain tissue microscopically. These areas appear normal grossly, and CT and MR imaging may not reliably define them (1). Pathologic studies have revealed tumor cells beyond regions of CT hypodensity and MR imaging T2 hyperintensity (32). The majority of studies have shown no effect of tumor volume on outcome. No correlation between the CT tumor volume estimated by cross-sectional area and survival was found among patients with glioblastoma multiforme (33–35). Correspondingly, MR imaging volumes have not been predictive factors in glio-blastoma multiforme (25) or anaplastic astrocytoma (27). These studies used a cross-product method to obtain an approximate enhancing volume. In contrast, the measurement method used in our study was more accurate, and small enhancing tumor volume on the pretreatment T1-weighted MR images was found to be associated with longer survival. Other parameters, such as edema, resection cavity, and combined volumes, were not predictive of outcome.
A high level of vascularity has long been recognized as an indicator of malignant potential, because it reflects degree of tumoral angiogenesis (36), which in turn reflects the tumor's ability to nourish the growing tumor mass. Angiogenesis is a prerequisite for solid tumor growth beyond a few millimeters (37). In high-grade tumors, endothelial cell proliferation—a key event in angiogenesis—is a common finding, whereas in low-grade tumors it is rare (38). Therefore, pathologic analysis aptly uses degree of vascularity as an important part of the determination between high- and low-grade lesions (39). In the World Health Organization classification system, anaplastic astrocytoma and glio-blastoma multiforme are differentiated from their low-grade counterparts by the presence of vascular proliferation (39).
Microvessel density, a histologic measure of vascularity, recently has been studied in patients with primary brain tumors. In 93 patients with anaplastic astrocytoma, glioblastoma multiforme and astrocytoma (five had astrocytoma), microvessel density was found to be an independent predictor of survival (39). In 74 patients with astrocytoma, microvessel density also was shown to be a prognostic factor for survival in low-grade tumors (40).
Another histopathologic method for evaluation of angiogenesis is measurement of the numerous growth factors thought to mediate angiogenesis. Vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), platelet derived growth factor (PDGF), fibroblast growth factor (bFGF), and transforming growth factor-β (TGF-β) have been shown to play a vital role in angiogenesis, and others also may be involved (41). Currently, however, it is not solidly established whether any one growth factor can be consistently predictive of survival. In low-grade astrocytoma, presence of VEGF was significant for survival while bFGF and EGF were not (40). In histologically varied gliomas (astrocytomas and oligodendrogliomas), EGF level was an independent predictor of disease-free survival (42).
One limitation of using histopathologic methods as a vascularity measure is that primary brain tumors characteristically are regionally heterogeneous (43, 44). In fact, tumor grades and histologic classifications are sometimes inaccurately identified when based on a small biopsy specimen (39). Therefore, for assessing vascularity, the small specimens obtained by stereotactic biopsy may not contain the necessary microscopic features.
In contrast, angiography allows a much larger volume of tumor to be visualized compared with histologic measures. Three-dimensional time-of-flight MR angiography has been used to evaluate pathologic vessels in 16 patients with malignant glioma (45). Displacement of major cerebral arteries and deep venous structures, feeding arteries, and draining veins were depicted almost as well by three-dimensional time-of-flight angiography as by conventional angiography. Yet conventional angiography further demonstrates functional aspects; abnormal flow patterns, such as arteriovenous shunting, can be observed. Because tumor microcirculation is heterogeneous, conventional angiography can show the varying intravascular flow rates from one region to the next (8, 46, 47). Furthermore, the hemodynamic pattern within a given region of a hypervascular tumor may change as the tumor grows, and subsequent angiograms may show the evolving appearance. Another advantage of angiography is that abnormal tumor blush can be compared with the normal surrounding parenchymal blush, and polymorphic vessels can be seen. Although angiography may be a crude measure of vascularity compared with histologic methods, angiographic findings of tumor hypervascularity have been correlated with tumors of greater malignancy, measured by nucleolar organizer regions, a reflection of cell activity (48). In our study, hypervascularity on the angiogram was a predictive factor for decreased survival. To our knowledge, this is the first study that has shown angiography to be significant for survival.
Conclusion
In this selected group of patients with recurrent glioblastoma multiforme and anaplastic astrocytoma treated with selective intra-arterial chemotherapy, two radiologic findings—large net tumor volume and hypervascularity—predicted poor prognosis. To our knowledge, this is the first time angiographic findings of vascularity have been demonstrated to be a significant prognostic factor in primary brain tumors. If confirmed by further studies, radiologic factors such as tumor volume and vascularity should be considered in addition to other previously established factors in designing future trials of intra-arterial chemotherapy.
Acknowledgments
We thank the following physicians who participated in this Phase II multicenter study for their help and input: Lee Guterman, DENT Neurological Institute; Gregory Joseph, Emory University; Vance Watson, Georgetown University; Frank Huan-Hellinger, Massachusetts General Hospital; Robert Eskridge, University of Washington; Christopher Moran, Washington University; and John Chaloupka, Yale University.
Footnotes
1 Supported in part by Alkermes Inc., Cambridge, MA.
2 Presented at the annual meeting of the American Society of Neuroradiology, Philadelphia, Pennsylvania, 1998.
↵3 Address reprint requests to Y. Pierre Gobin, MD.
References
- Received June 1, 1999.
- Copyright © American Society of Neuroradiology