Abstract
BACKGROUND AND PURPOSE: Patients with spontaneous intracerebral hemorrhage (ICH) frequently have small areas of signal loss on gradient-echo T2*-weighted MR images, which have been suggested to represent remnants of previous microbleeds. Our aim was to provide histopathologic support for this assumption and to clarify whether the presence and location of microbleeds were associated with microangiopathy.
METHODS: We performed MR imaging and correlative histopathologic examination in 11 formalin-fixed brains of patients who had died of an ICH (age range, 45–90 years).
RESULTS: Focal areas of signal loss on MR images were noted in seven brains. They were seen in a corticosubcortical location in six brains, in the basal ganglia/thalami in five, and infratentorially in three specimens. Histopathologic examination showed focal hemosiderin deposition in 21 of 34 areas of MR signal loss. No other corresponding abnormalities were found; however, hemosiderin deposits were noted without MR signal changes in two brains. All specimens with MR foci of signal loss showed moderate to severe fibrohyalinosis, and there was additional evidence of amyloid angiopathy in two of those brains.
CONCLUSION: Small areas of signal loss on gradient echo T2*-weighted images indicate previous extravasation of blood and are related to bleeding-prone microangiopathy of different origins.
Spontaneous intracerebral hemorrhage (ICH) is primarily caused by the rupture of small and medium-sized arteries. Hypertension-induced fibrohyalinosis is the most frequent type of vessel wall damage to trigger such an event (1, 2). Extensive deposition of β-amyloid within the walls of small vessels has been recognized as another important cause of increased vascular fragility (3, 4). In addition to creating a higher risk for ICH, both types of angiopathy favor the development of ischemic damage to the brain. Respective tissue changes, such as leukoaraiosis and a lacunar state of the basal ganglia, are therefore frequently observed in patients suffering from ICH, and their presence has been suggested to indicate a higher risk for cerebral hemorrhage (5, 6).
In this context, attention has also focused on MR imaging because of its potential to reveal residue of intracerebral hemorrhage throughout life. After an ICH, hemosiderin remains stored in macrophages and, because of its magnetic properties, leads to focal dephasing of the MR signal, causing hemosiderin-containing areas to appear dark on T2-weighted spin-echo sequences. This effect may be further enhanced by the use of imaging techniques with high sensitivity for differences in magnetic susceptibility, such as the gradient-echo sequence (7). Offenbacher et al (8) found multiple intracerebral foci of MR signal loss in 28 of 120 patients with ICH. In the absence of any other explanation for such signal changes, these lesions were assumed to represent remnants of previous, clinically silent, microbleeds. They were seen in different regions, such as in the basal ganglia and thalami corticosubcortically, but also in infratentorial structures of the brain (8). At the same time, Greenberg et al (9) observed similar changes in nine of 15 patients with a lobar hemorrhage. These abnormalities were also considered as evidence of previous petechial hemorrhages and, because of their location preferentially at corticosubcortical sites, were thought to be associated with amyloid angiopathy.
To provide histopathologic support for these assumptions, we performed postmortem MR imaging on the brains of 11 patients who had died of an ICH. Our major goals were to confirm that focal areas of signal loss on gradient-echo MR images corresponded to areas of previous extravasation of blood, and to clarify whether their presence or location was associated with a specific type of microangiopathy.
Methods
We studied the brains of 11 patients who had consecutively come to autopsy after death caused by an ICH between May 1995 and April 1996. The patients' ages (mean age, 72 years) and the presence of hypertension are listed in Table 1. Hypertension was defined by a history of increased blood pressure (> 160/95 mm Hg) or medical treatment for hypertension. Three patients (cases 1, 2, and 5) had a history of stroke. One patient (case 5) had been on a diet for diabetes mellitus. At postmortem, the brains were removed in total and fixed in 10% formaldehyde solution for at least 3 weeks before scanning.
Demographic data and MR findings
MR imaging was performed on 1.5-T scanners. We obtained gradient-echo T2*-weighted images in the axial plane with the following parameters: 550–650/15/2 (TR/TE/excitations), a flip angle of 25°, a section thickness of 5 mm with a gap of 0.5 mm, and a matrix of 256 × 205. Axial spin-echo T1-weighted images (500–600/15/2) and fast spin-echo T2-weighted images (2200–3200/80–120/1,2; turbo factor, 24) were also obtained with the same section thickness and matrix. An experienced MR interpreter who was unaware of the clinical data reviewed all images for type and location of ischemic and hemorrhagic lesions before histopathologic work-up. Focal areas of signal loss with a diameter of up to 5 mm were termed microbleeds (8). Lesions with the typical appearance of an old hematoma were recorded separately. Areas of supposedly parenchymal ischemic destruction with a diameter of less than 10 mm were termed lacunae. Signal hyperintensity in the centrum semiovale was graded as punctate (grade 1), patchy/early confluent (grade 2), or confluent (grade 3) (10). Patients 1 and 9 had undergone MR imaging prior to death, and these images were compared with the postmortem studies.
The technique adopted for correlating MR and histologic sections followed previous recommendations (11). In short, cutting of the fixed specimen was guided by the sagittal MR scout view to obtain 5-mm-thick sections parallel to the imaging plane. Sections containing areas with MR lesions served to prepare either gross hemispheric (n = 34) or selected microscopic (n = 11) sections. Sections were stained with hematoxylin-eosin, Masson trichrome, the Klüver Barrera technique for myelin, Congo red for amyloid, and with iron. First, the histopathologic data were tabulated by a neuropathologist who was blinded to the MR findings. He recorded the type and severity of microangiopathy and the type and location of ischemic and hemorrhagic brain lesions. Small-vessel sclerosis and fibrohyalinosis were rated as mild, moderate, or severe (associated with marked ischemic tissue damage), and the severity of changes attributable to amyloid angiopathy was graded accordingly (12). Thereafter, we reviewed MR and histologic sections in parallel to determine the extent of correlation between imaging and pathologic findings.
Results
The terminal ICH was lobar in seven patients and was located in the thalamus/basal ganglia in three and in the brain stem in one. Gradient-echo T2*-weighted MR images showed focal areas of signal loss outside the terminal ICH in seven brains (Fig 1A and B). The number and location of these hypointensities are shown in Table 1. Only a few of these foci would have been detectable on fast spin-echo T2-weighted images. In patient 1, two possible microbleeds were seen on in vivo images but could not be identified on images of the brain specimen. Conversely, in patient 9, a focal area of signal loss was noted on the postmortem MR images only. There was evidence of a previous hematoma in two patients and old territorial infarcts were noted in another one. Lacunae predominantly of the basal ganglia were suspected in seven specimens. A clear definition of lacunar infarcts on postmortem MR images was complicated by fixation-induced signal intensity changes on the T1-weighted sequence (11), and areas of signal hyperintensity in the centrum semiovale were rather poorly delineated on postmortem images.
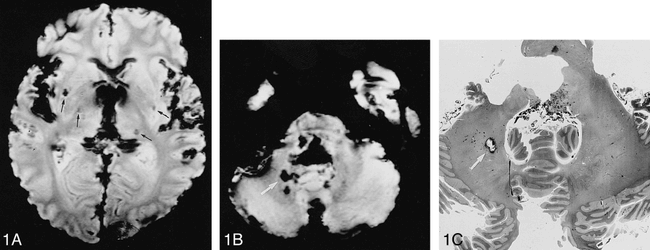
Patient 6.
A and B, Postmortem gradient-echo T2*-weighted MR images (600/15/2; flip angle, 25°) show foci of signal hypointensity in the basal ganglia bilaterally and in the left thalamus (arrows, A). Note signal loss at the surface of the specimen due to field inhomogeneity and subarachnoid blood. Foci of signal loss are also noted in the cerebellum and pons (arrow, B).
C, Histopathologic section shows an old microbleed (arrow), corresponding to the largest hypointensity (corresponding arrows in B and C).
Histopathologic findings are listed in Table 2. Focal accumulation of hemosiderin-containing macrophages was seen in 21 of 34 areas with signal loss on MR images (Fig 1B and C). Such deposits were most frequent in the basal ganglia adjacent to small blood vessels and sometimes associated with minute areas of tissue necrosis. For the remaining MR hypointensities, no specific pathologic substrate was found. Hemosiderin deposits were also noted without MR signal changes in two brains and could not be confirmed in one brain with two foci of signal loss. MR-negative hemosiderin deposits tended to be smaller and consisted of only a few perivascular, hemosiderin-laden macrophages. There was no evidence of calcification or vascular malformations, such as a cavernous hemangioma, at any of the sites examined. MR findings suggestive of lacunae of the basal ganglia and thalami were confirmed in five of seven brains. Two lacunae were noted in another brain, and a cribriform state was present in eight specimens. Areas of MR white matter hyperintensity corresponded to rarefaction of myelin staining in five of eight brains and were associated with diffuse white matter edema in one specimen. Lacunae or extensive white matter damage were not strictly correlated with either MR hypointensities or focal hemosiderin deposits.
Histopathologic findings
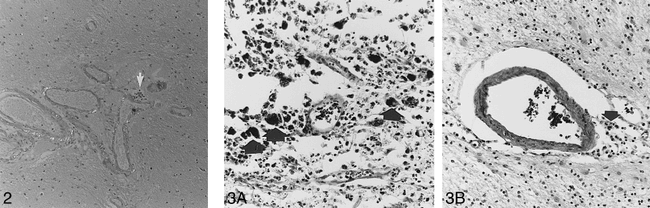
There was evidence of moderate to severe fibrohyalinosis in all specimens from the patients with hypertension (Table 1). The brains of patients 1 and 2 were also positive for cerebral amyloid angiopathy. Amyloid deposition was variably extensive, with replacement of the media in multiple vessels. These changes were associated with foci of remote blood leakage in both specimens (Fig 2). Patient 1 had had a previous hemorrhage. In the brain of patient 2, old microbleeds were also seen in the basal ganglia. Neuritic plaques and neurofibrillary tangles were absent. Brains with fibrohyalinosis showed microbleeds preferentially in the basal ganglia and thalami, but foci of blood leakage were also observed in a corticosubcortical distribution in two of them (Fig 3).
Cerebral amyloid angiopathy in area showing hypointense MR lesion. Yellow-green birefringency of amyloid deposits in the vessel walls under polarized light corresponds to bright spots and perivascular leakage of erythrocytes (arrow). Temporal cortex, polarization optics; alkaline Congo stain, original magnification ×32.
fig 3. A, MR-positive old microbleed with a diameter of 2 to 3 mm in the subcortical white matter. Numerous darkly stained hemosiderophages are seen in the center, close to a ruptured vessel (arrows). There is extensive perivascular edema. Masson trichrome stain, original magnification ×201.
B, Hypertensive angiopathy with dilatation of perivascular spaces and deposition of a few hemosiderin-laden macrophages (arrow) not seen on MR study. Reactive astrocytes are present in the surrounding neuropil. Masson trichrome stain, original magnification ×162.
Discussion
This study lends support to the assumption that focal areas of signal loss on gradient-echo T2*-weighted MR images of patients with ICH are indicative of past microbleeds. Histopathologic examination frequently showed hemosiderin deposits in areas with signal loss on MR images, and there was no evidence of other possibly related morphologic abnormalities, such as focal calcification or small vascular malformations. However, the correlation between MR hypointensities and hemosiderin deposits was not absolute: histopathologic examinations of some regions with MR lesions were negative and a few hemosiderin-containing macrophages were noted around small blood vessels without corresponding signal changes on postmortem MR studies. The small size of both MR hypointensities and their histopathologic correlate is the most likely cause of our failure to find corresponding abnormalities for all MR lesions. When visual inspection fails to reveal overt damage of the brain sections, which guides the selection of regions for histopathologic examination, some topographic mismatch between MR and microscopic sections cannot be avoided. In some instances, the amount of hemosiderin deposition and related field inhomogeneities may not have been large enough to become apparent on MR images. Because we just wanted to define the histopathologic substrates associated with focal MR hypointensities in patients with ICH, we did not perform a systematic search for MR-negative hemosiderin deposits throughout all brains. Therefore, we cannot report how often and below which threshold of hemosiderin deposition previous microbleeds may be missed, even when using gradient-echo T2*-weighted sequences. The much higher sensitivity of this sequence for detecting previous microbleeds than that of conventional or fast spin-echo T2-weighted imaging has been documented in clinical series (9, 13, 14) and was confirmed by this study.
Differences in the detection of MR hypointensities on pre- and postmortem MR images of two brains probably were a consequence of minor variations in section orientation. However, some change in sensitivity of the gradient-echo T2*-weighted sequence for hemosiderin deposits with fixation of the brain cannot be excluded. Field inhomogeneities from subarachnoid blood at the surface of the brain on postmortem MR images may have contributed to a lower conspicuity of minute hypointensities.
All brains examined showed moderate to severe small-vessel disease. Overall, seven patients had been hypertensive and there was a history of hypertension in five of seven patients with MR foci of hypointensity. Areas of signal loss in the brains of these individuals were located predominantly in the basal ganglia, thalami, brain stem, or cerebellum. More recently, Chan et al (13) reported multifocal hypointense cerebral lesions on gradient-echo MR images in patients with chronic hypertension. Our findings corroborate this association and provide the histopathologic basis for their observation; that is, remnants of blood seepage through damaged arteriolar walls.
Cerebral amyloid angiopathy was noted in two of our specimens. One of them showed a purely corticosubcortical location of previous petechial hemorrhages in addition to an old lobar hemorrhage. This pattern has been proposed as strongly suggestive of amyloid angiopathy (8, 12). However, old microbleeds in a corticosubcortical location were also seen in some of our patients with hypertensive microangiopathy, as in the series of Chan et al (13). Conversely, the presence of microbleeds in the basal ganglia and thalami in addition to corticosubcortical hemosiderin deposits does not exclude the presence of amyloid angiopathy, as seen in another of our specimens. Widespread involvement of arterioles by both types of angiopathy and coexistence of both pathogenic mechanisms in at least some individuals are likely causes for some overlap in the distribution of microbleeds.
The high number of concomitant ischemic lesions observed histopathologically confirms previous notions of a higher risk for cerebral hemorrhage in patients with lacunar infarctions and advanced ischemic white matter damage (5, 6). We also noted some difficulties when interpreting postmortem MR images in this regard. Extensive hyperintensity throughout the centrum semiovale, potentially indicating white matter rarefaction, frequently could not be differentiated from diffuse edema caused by increased intracranial pressure. There was also a tendency for interpreting a cribriform state of the basal ganglia as consistent with multiple lacunar lesions.
Conclusion
Even our limited sample of brain specimens allows support of a strong association between ICH and focal areas of signal loss on gradient-echo T2*-weighted MR images. Moreover, MR evidence of past microbleeds appears to be a direct marker of increased vascular fragility in patients with various types of small-vessel disease (15). Therefore, such abnormalities may be a better predictor of a patient's risk for hemorrhage than are clinical findings, like hypertension, or CT changes of leukoaraiosis (16). In this context, MR imaging might help in selecting patients for different types of secondary prevention of stroke. More recently, a trial of anticoagulation following transient ischemic attacks had to be terminated prematurely because of an excessively high rate of spontaneous intracerebral hematoma (16). Possibly, MR evidence of microbleeds could serve to identify patients at risk for such a complication. Further testing of this hypothesis appears warranted.
Footnotes
↵1 Address reprint requests to Franz Fazekas, MD, Department of Neurology, Karl-Franzens University, Auenbruggerplatz 22, A−8036 Graz, Austria.
References
- Received July 17, 1998.
- Copyright © American Society of Neuroradiology