Abstract
BACKGROUND AND PURPOSE: Temporary balloon occlusion has become a routine and medically accepted technique for the management of patients with aneurysms or intracranial or head/neck tumors. We describe our experience using a nondetachable silicone balloon (NDSB) catheter in 103 endovascular temporary balloon occlusions of the internal carotid artery, with attention focused on technique, complications, and cost.
METHODS: Between 1993 and 1998, 103 patients underwent preoperative temporary balloon occlusion testing with a 1.5-mm NDSB catheter. Clinical testing during endovascular blockade was combined with qualitative cerebral blood flow analysis using technetium-99m HMPAO SPECT. Cost-effective analysis was performed, emphasizing cost and complication rates in comparison with those in previously reported series in which multiple types of temporary balloon occlusion catheters were used, predominantly not of the NDSB type.
RESULTS: No carotid artery injury or complication, including cerebral infarction due to NDSB use, was encountered. Despite the increased cost of the NDSB catheter system, cost-effective analysis showed up to 40% reduction in cost per quality adjusted life years.
CONCLUSION: Temporary balloon occlusion using the NDSB catheter is safe and cost-effective, owing to the low rate of complications.
Temporary balloon occlusion has become a routine and medically accepted technique for the management of patients with aneurysms or intracranial or head/neck tumors who will likely require prolonged intraoperative or permanent occlusion of the internal carotid artery (ICA). The purpose of this study was to add to the growing body of evidence supporting the safety and efficacy of temporary balloon occlusions, highlighting the use of a nondetachable silicone balloon (NDSB) system to minimize the risk of procedural complications, including vascular injury and stroke.
Methods
Procedure
Baseline neurologic and physical examinations were performed. Patients were usually referred with preexisting imaging studies, including CT or MR imaging. If not already available, diagnostic arteriography was initially performed to evaluate the carotid and vertebral arteries through a 5F femoral arterial sheath with a 5F diagnostic, single end-hole catheter. (Diagnostic arteriography assesses the status of the carotid bifurcations, ICAs, and intracranial circulation, and helps to determine the need for an 8F guiding catheter to assist balloon catheter deployment.) A 6F femoral sheath was then placed in the opposite femoral artery for balloon catheter passage. The 5F sheath was maintained for diagnostic catheter injections during the balloon occlusion test to evaluate innate cerebral collaterals.
The NDSB catheter (Target Therapeutics, Fremont, CA) is prepared with a 0.010 Seeker guidewire through a Touhy-Borst adapter (Target Therapeutics), and 5000 to 10000 U of heparin is administered intravenously, increasing activated coagulation time (ACT) to three times its baseline value. The catheter system is passed directly through the 6F sheath into the ascending aorta. A short segment of another 5F sheath may be used as an introducing piece, facilitating insertion of the NDSB through the 6F sheath's diaphragm. When inflated in the ascending aorta, the NDSB catheter typically enters the innominate artery. For cannulation of the right common carotid artery (CCA), this approach was used in 53% of cases, failing in only one case when attempted. Depending on the origin and orientation of the proximal left CCA, direct catheterization using the NDSB without an 8F femoral sheath and guiding catheter was done in 36% of patients, failing in only one case when attempted. If initial attempts to directly catheterize the left CCA with an inflated NDSB are not successful, inflation of the balloon in the ascending or descending aorta to form a “U” configuration to the catheter often allows flow-directed selection. In patients with significant aortic dilatation, elongation, or great-vessel tortuosity, a guiding catheter, such as the Schneider Judkin Type 4, may be passed primarily or by exchange into the arch origin of the left CCA with coaxial passage of the balloon catheter. With experience, a guiding catheter is likely to be needed in no more than 10% to 20% of cases.
Under fluoroscopic guidance, the balloon tip is preferentially passed to and inflated within the petrous segment of the ICA, easily traversing tortuous, even kinked, segments of the cervical ICA. The petrous segment is a fixed segment of the ICA considered to have a low risk of vessel-wall injury. The balloon is then carefully inflated under fluoroscopic guidance with manual traction exerted to prevent distal migration of the balloon tip. Inflation is performed under fluoroscopic visualization looking for slight elongation and position stability during injection of 0.15 to 0.25 mL dilute contrast material. Complete vessel occlusion is then presumed; it may be confirmed, if necessary, by proximal contrast injection through a guiding catheter, if present, or a tandem diagnostic catheter. A static column of contrast material indicates complete carotid occlusion. Stagnating contrast in the occluded vessel may be thrombogenic. Subsequent heparinized saline flush through the 8F guidecatheter or proximally positioned 5F diagnostic catheter helps to clear residual contrast from the proximal vessel throughout the remainder of the procedure. Alternatively, the balloon may be deflated to clear the vessel of any remaining contrast, then reinflated. If the patient initially tolerates test occlusion by clinical examination, then diagnostic arteriography in a vertebral or subclavian artery, and contralateral carotid artery is performed to qualitatively evaluate collateral flow. The angiographic catheter is then placed in the proximal CCA on the side of ICA occlusion, and heparinized flush is injected for the remainder of the catheterization.
Continuous neurologic testing throughout 20 to 30 minutes of temporary occlusion excludes early evidence of ischemia. Should the neurologic examination deviate from baseline, the temporary occlusion procedure must be terminated. For those patients tolerating the initial 10-minute occlusion by clinical evaluation, qualitative technetium-99m HMPAO SPECT is performed. Injection of the radiopharmaceutical is done during balloon occlusion, allowing 5 to 10 minutes of isotope circulation time during ongoing occlusion. If the patient tolerates occlusion at normotension, an additional challenge to cerebral perfusion may be performed by the anesthesiologist using sodium-nitroprusside infusion: mean arterial pressure is reduced to 75% of its baseline level by continuous arterial pressure monitor. At the end of testing, a CCA injection is done to evaluate external carotid artery to ICA collaterals. The balloon catheter is then deflated and removed, normotension is restored, and the patient is taken to the nuclear medicine section for cerebral SPECT. The role of xenon CT (1–3) is currently under evaluation at this institution for which the low-profile balloon catheter may be left in position or deflated and then reinflated to the identical filling volume identified previously for 10 minutes in the CT scanner during inhalation/equilibration of inert xenon gas. Postprocedural ACT is obtained before the femoral arterial sheath is removed. Protamine (10 mg/1000 U heparin) may be administered if the ACT remains greater than 180 seconds.
Cost-Effectiveness Analysis
Cost-effectiveness analysis compares the marginal cost to use the NDSB catheter system with the use of the least expensive balloon catheter system described in temporary balloon occlusion testing of the ICA. We compared the excess equipment cost of the NDSB system associated with the de facto complication rate, identified as completed cerebral infarction in this series, with the theoretical cost of cerebral infarction incurred in this and other reported series in which less expensive catheter systems were used.
Cost-effectiveness analysis was performed using methodology described previously (4). Average historical rates of acute complication for the temporary balloon occlusion procedure using various catheter systems were calculated over a 25-year life expectancy based on a patient's age of 52 years, the mean patient age in our series of parent vessel occlusions for ICA aneurysms. The costs of complications resulting from stroke, quality of life changes, follow-up care, and monetary discount effects were considered. Charge data for patients at this institution were converted to cost data using Medicare cost-to-charge ratios (5). Stroke treatment cost is estimated on the basis of published data (6, 7), assuming 40% of patients surviving stroke require inpatient rehabilitation and 15% require nursing home care. Discount effects incorporated into the model account for the time-value of money. To account for the effect of time, charges for care and cost per quality-adjusted life year (QALY) were discounted using the 5% discount rate previously used in other analyses for this purpose (6). Numerical results are reported in standardized units QALY, according to the following formula:
Cost/QALY, where cost = [{initial costs) + (nth year follow-up costs) + {(complication A costs) × (probability of complication A in year n)} + . . . + {(complication Z costs) × (probability of complication Z in year n)}]/[(1 +r)n] where n = 1 through 26 years of life expectancy, r = discount rate, and initial costs occur in first year only; and QALY = [{(QOL rating of outcome A) × (probability of outcome A in year n)} + . . + {(QOL rating for outcome Z) × (probability of outcome Z in year n)}]/[(1+r)n]
where QOL indicates quality of life. Professional charges and indirect costs were not included.
Results
Between 1993 and 1998, 103 temporary balloon occlusions of the ICA were performed at two institutions. No ICA dissections or permanent ischemia due to the NDSB were encountered. A permanent neurologic deficit related to the arteriographic component of the procedure occurred in a 61-year-old woman (n = 1; 0.97%). In this patient, left ICA balloon occlusion testing included routine right CCA arteriography. Recatheterization of the right CCA with hyperextension of the neck for a submentovertex projection was also performed to evaluate a suspected anterior communicating artery aneurysm. Thirty-six hours after balloon occlusion testing of the left ICA for a giant aneurysm, the patient exhibited confusion, agitation, and left hemiparesis. Repeat arteriography showed long-segment intimal dissection of the right ICA with near occlusion of the vessel lumen. This patient subsequently underwent right superficial temporal to middle cerebral artery bypass graft placement with partial resolution of deficits.
A 73-year-old woman experienced transient confusion and agitation 20 to 30 minutes after deflation of the NDSB balloon catheter and transfer to the radioisotope laboratory for scanning. Clinical examination was nonfocal. Although the period of agitation interrupted the usual sequence of events, including the scheduled SPECT examination, the patient regained normal mental status and had no CT evidence of ischemia or hemorrhage with supportive care alone.
Cost-effective analysis discloses that excess equipment cost to use the NDSB system is not the primary determinant of cost in the balloon occlusion test. The isolated cost of the temporary balloon occlusion procedure increases because of the routine use of the NDSB system over less expensive alternatives, such as the 5F Swan-Ganz catheter (Edwards Laboratories, Puerto Rico). Because of its small diameter (2F) and overall compliance, the NDSB system (Target Therapeutics, $425.00) does not lend itself to custom shaping and requires a 0.010 guidewire (eg, Seeker Lite, Target Therapeutics, $140.00) for stabilization and passage against arterial flow in the aorta. If the NDSB does not readily pass directly or indirectly with blood flow into the vessel of choice, an 8F guiding catheter (Schneider JR4, $105.00) may be used. Compared with the low unit cost of a Swan-Ganz catheter ($21.50 at this institution), the incremental procedure equipment cost ranges from $543.50 to $670.00 per procedure. Another commonly used balloon occlusion device is the MIS 5.41F Zeppelin balloon catheter ($280.00), although it is no longer available.
Cost per QALY to use the NDSB system is $113.51 in our experience, based on a 1.0% stroke complication rate. Use of less expensive temporary occlusion catheters, especially the least expensive Swan-Ganz catheter at $21.50 per unit, results in a cost of $267.01 per QALY based on the 0% to 8.3% reported range of ischemic complications, with an average stroke rate of 3.3% (8–14). Berg-Dammer et al (15) reported a series of thromboembolic occlusions of the middle cerebral artery due to angiography and endovascular procedures. Although the total number of balloon test occlusions and the type of occlusion balloon systems are not specified in their series, five (5.7%) of 88 procedural ischemic complications requiring emergent intraarterial thrombolysis were due to balloon occlusion procedures. Horowitz et al (16) reported a 5.7% complication rate, including one fatality predominantly due to cerebral ischemia, in 88 temporary balloon occlusion procedures performed between 1993 and 1996. At the highest complication rate reported by Simonson et al (10) (ie, 8.3%), cost per QALY would range as high as $650.04 even when the least expensive equipment is used. As one might expect, cost per QALY varies more with the complication rate than with marginal equipment cost.
Sensitivity analysis indicates that the marginal equipment cost of the NDSB system would have to increase to approximately $2711 (an increase of 404% to 499%) to achieve cost per QALY parity with the theoretical model for the least expensive system at a 3.4% rate of ischemic complications. Similarly, the stroke rate associated with the NDSB system would have to increase to 3.0% to reach cost/QALY parity with less expensive systems. Conversely, the reported complication rate of temporary balloon occlusion procedures with non-NDSB systems would have to decrease to 1.4% to reach cost/QALY parity with our NDSB experience.
Tables 1 through ⇓3 provide comparable data points using the theoretical mathematical model to compare cost/QALY associated with the use of the NDSB and other systems at various rates of ischemic complications. The product of the equation represents cost: NDSB system (cost/QALY) minus other temporary balloon occlusion systems (cost/QALY) at various rates of permanent neurologic complication due to cerebral infarction.
NDSB system marginal cost of $543.50 (NDSB and 0.010 guidewire vs least expensive system at various complication rates)
NDSB system marginal cost of $670.00 (NDSB, 0.010 guidewire, and guidecatheter vs least expensive system at various complication rates
MIS Zeppelin system marginal cost of $280.00 (balloon catheter only vs least expensive system at various complication rates)
Discussion
Comments on the Temporary BalloonOcclusion Technique
In 216 endovascular temporary ICA occlusions performed at two institutions, 103 procedures between 1993 and 1998 were done using the Heishima or Tracker 18 mounted NDSB catheter (Target Therapeutics, items 781512, 781552). The most compliant of the nondetachable silicon balloon occlusion catheters, the NDSB requires the least inflation pressure, minimizes inflation wall pressure, and its 2F shaft more easily traverses tortuous vessels than many alternative devices (17). Variable combinations of the NDSB with introducing sheaths, guidecatheters, and guidewires were applied in this series, depending, in part, on the patient's anatomy.
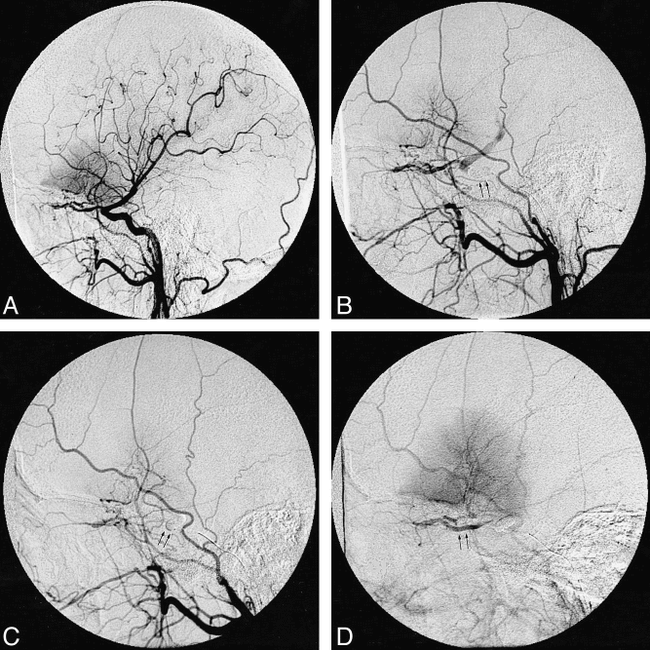
An additional benefit of the NDSB that bears mention is the occasional need for intradural balloon occlusion testing. Under certain circumstances, in which significant extracranial to intracranial arterial collaterals may play an important role in cerebral perfusion, balloon occlusion testing is likely to be accurate only if sources of collateral vascular supply, such as the ophthalmic artery, can be blocked concurrently with the ICA (Fig 1). Significant collateral vessels may be found in association with head and neck neoplasms or long-standing vascular stenosis.
58-year-old woman with left planum sphenoidal meningioma undergoing preoperative temporary balloon occlusion test with the NDSB system.
A, Digital radiograph, arterial phase, during left CCA injection, shows neovascular tumor blush above planum sphenoidal and mild depression and narrowing of the supraclinoid ICA segment, indicating partial vessel encasement by tumor, also apparent by MR imaging.
B, Digital radiograph, arterial phase, during left CCA injection after inflation of NDSB (arrows) in cavernous ICA segment, shows prominent retrograde collateral opacification of the ophthalmic artery, supraclinoid ICA, and contribution to MCA flow.
C and D, Sequential digital radiographs, mid and late arterial phase, during left CCA injection after inflation of NDSB (arrows, C) in ophthalmic segment of left ICA, show successful exclusion of retrograde ophthalmic artery contribution (arrows, D) to the left ICA circulation.
On a case-by-case basis, one of two 1.5-mm NDSB catheters is selected. For routine balloon test occlusion performed with inflation of the balloon in the petrous segment of the ICA, a 0.5-cm3, 1.5-mm balloon tip catheter is chosen. At maximum inflation with 0.5 cm3, maximum balloon dimensions measure 7.5 × 13.5 mm. Although maximum inflation is never required for temporary flow occlusion of the petrous ICA, inflation toward maximum may at times be useful during flow-guided first-order selection. Alternatively, when preoperative evaluation indicates the presence of significant carotid stenosis or a need for more distal intracranial, clinoidal, or supraclinoid test occlusion, as noted previously, the 0.1-cm3 1.5-mm NDSB with maximum balloon inflation of 5.0 × 6.0 mm may be an appropriate choice.
Complications
No arterial wall dissections or infarcts due to the NDSB were identified. The only ischemic complication (n = 1; 0.97%) that occurred in this series of 103 patients who underwent temporary balloon occlusion was unrelated to NDSB use. Latent carotid dissection contralateral to a large carotid cavernous aneurysm resulted in subtotal vessel occlusion and hemispheric ischemia, possibly attributable to subintimal guidewire passage through a diagnostic catheter that was not apparent at the time of the balloon occlusion test.
A second patient became agitated for undocumented reasons after the temporary balloon occlusion procedure. Confusion and agitation began 20 to 30 minutes after the balloon catheter was deflated and removed. In retrospect, the ACT value had reached an appropriate level of anticoagulation for the balloon occlusion test and had not been reversed with protamine. The patient's temporary inability to cooperate precluded the brain SPECT examination. With only supportive care, this patient recovered without further incident or imaging aberration to suggest brain ischemia or hemorrhage.
The single permanent procedure-related complication in this intermediate-size series, although unrelated to the balloon catheter itself, compares favorably with the cumulative complication rate reported by other investigators between 1987 and 1995. Complication rates previously reported range between 0% and 8.3% in a cumulative total of 769 temporary balloon occlusion procedures. In the single largest series of 500 cases, Mathis et al (18) divided their 3.2% complication rate evenly between asymptomatic persons and symptomatic persons with neurologic deficits, 0.4% of which progressed to permanent deficits. Although Mathis and coworkers noted that the Swan-Ganz catheter was the most commonly used balloon occlusion device in their series, they do not specify which catheter systems were associated with the complications they encountered.
Implications of Temporary Balloon Occlusion with the NDSB Catheter
Temporary balloon occlusion has become an established method for predicting the relative risk of stroke after prolonged intraoperative temporary occlusion or surgical ablation of the ICA. Unprotected acute carotid occlusion results in a cumulative cerebral infarction rate between 26% and 100% (19, 20). Because abrupt ICA occlusion in the unscreened population may result in infarction of a large zone within the anterior circulation, a mortality rate of 12% after vessel occlusion has been cited (18). By contrast, patients who pass a temporary balloon occlusion procedure with clinical testing alone have only a 4.7% stroke risk, with 0% mortality. Linskey et al (19) reported that the risk of stroke fell to 3% with the addition of xenon CT cerebral blood flow analysis in their large series.
Lawton et al (21) have argued against temporary balloon occlusion testing for aneurysms in favor of universal revascularization using the bypass graft with parent vessel occlusion technique for any patient requiring prolonged intraoperative occlusion or ICA sacrifice, particularly for ICA aneurysms. Their prescribed treatment plan obviates temporary balloon occlusion testing, as each applicable patient undergoes bypass graft placement at the time of permanent surgical occlusion of the parent internal carotid vessel. They argue that the balloon occlusion test only adds unnecessary cost and risk to their protocol. More recent cost analysis, however, has revealed greater cost efficiency of the endovascular treatment approach. A comparison of acute treatment cost, including the cost of bypass graft with parent vessel occlusion versus temporary and permanent balloon occlusion, in addition to the outcome cost of acute stroke management, has shown the theorectical cost efficiency of the endovascular approach with a 23% reduction in cost per QALY (4).
Justification of additional cost in this procedure can be made conceptually and quantitatively. Incremental cost of NDSB use accrues linearly. That is, the marginal cost per procedure is relatively fixed and increases linearly with case volume. By contrast, the cost of procedurally related complications, such as stroke, begins to accrue nonlinearly; that is, exponentially, becoming a renewable expense as long as each disabled patient lives.
The initial equipment cost at a given institution may exceed the cost of complications, especially in a small series, as probability would have it. However, a single procedural infarct in a patient with expected longevity of 25 years of disability may justify the marginal equipment cost in nearly 400 temporary balloon occlusion cases (5, 6). Thus, use of the NDSB system can be supported at centers with both large and small volumes of temporary balloon occlusion cases but for different reasons. At a referral institution with a high volume of temporary balloon occlusion procedures, use of the NDSB may help reduce the statistical probability of permanent complications in a large cohort of patients. At a smaller institution with relatively infrequent temporary balloon occlusion procedures, use of the NDSB likely offers an extra margin of safety in less familiar hands.
Conclusion
Use of the NDSB catheter for temporary endovascular occlusion of the ICA may reduce the risk of balloon catheter-related complications of vessel injury and cerebral infarction. Although the individual procedural equipment cost is increased, cost-effective analysis shows that prevention of a single complication resulting in clinically significant cerebral infarction substantially offsets that marginal increase.
Footnotes
↵1 Address reprint requests to Thomas A. Tomsick, MD, FACR, Department of Radiology, University of Cincinnati Medical Center, 234 Goodman St, Mail Location 742, Cincinnati, OH 45267.
References
- Received June 27, 1998.
- Copyright © American Society of Neuroradiology