Abstract
Summary: Diffusion-weighted MR imaging is generally thought to be highly sensitive for the diagnosis of acute stroke. We report two cases of hyperacute stroke with absence of changes on diffusion-weighted images within 4 hours of symptom onset. Follow-up studies, performed 4 days later, showed infarction in regions compatible with the clinical presentation and (in one case) with the initial perfusion deficit. These cases indicate that normal findings on diffusion-weighted images in patients with suspected cerebral ischemia do not rule out impending infarction.
Since the description by Moseley et al (1) of a 30% to 50% decrease of water diffusion constant in acute cerebral feline ischemia, diffusion-weighted MR imaging has been shown to be useful for the early diagnosis of stroke in humans. Diffusion-weighted imaging has been found to be much more sensitive than conventional T2-weighted MR imaging or CT in the detection of early changes associated with hyperacute stroke (<6 hours) (2), with a sensitivity as high as 100% (3). In the study with the largest number of participants examined with diffusion-weighted imaging within 6 hours of symptom onset, positive findings were reported in 32 of 34 infarctions (94% sensitivity) and negative findings in 14 of 14 patients without stroke (100% specificity) (4).
We describe two patients with symptoms of acute stroke (≤ 4 hours after onset) who had normal findings on diffusion-weighted images that progressed to infarction on follow-up imaging studies. These cases demonstrate that a negative diffusion-weighted image alone does not rule out a diagnosis of cerebral ischemia and the potential progression to infarction. The prevalence of normal findings on diffusion-weighted images in the earliest stages of human stroke may be higher than previously thought.
Patients and Methods
Ten patients with radiologically and clinically proved acute stroke were assessed by MR imaging within 24 hours of symptom onset between October 17, 1997, and September 9, 1998, at Johns Hopkins Hospital. Three of these patients had negative findings on initial diffusion-weighted images; two of these patients are reported in detail below.
MR imaging was performed on a 1.5-T MR unit equipped with echo-planar capability. Conventional spin-echo sagittal T1-weighted images (535/10/1 [TR/TE/excitations]) were obtained with a 24-mm field of view (FOV) and 5-mm-thick sections with a 1-mm gap. Axial double-echo spin-density and T2-weighted images (3000/30,100/0.75) were obtained with a 24-mm FOV, 5-mm-thick sections, a 256 × 192 matrix, flow compensation, and variable bandwidth.
Axial diffusion-weighted images were recorded using a single-shot spin-echo echo-planar pulse sequence (3000/100, 128 × 128 matrix, 24-cm FOV, 5-mm-thick sections with a 2.5-mm gap) with seven diffusion gradients, each applied in three directions (b-values = 10, 42, 169, 333, 490, 679, and 822 s/mm2). Isotropic diffusion-weighted images and average diffusion coefficient maps, Dav = (Dxx + Dyy + Dzz)/3, were produced off-line (5).
Perfusion MR imaging was performed in one case using a bolus injection of contrast material during continuous acquisition of a gradient-echo spiral pulse sequence (55/35, 64 × 64 matrix, 24-cm FOV, 5-mm-thick sections with a 2.5-mm gap). This sequence provides whole-head coverage with 17 section locations at 1-second time resolution (6). The sequence was repeated 60 times, for a 1-minute scan time. Gadopentetate dimeglumine (0.15 mL/kg) was injected into the antecubital vein using a power injector at 5 mL/s, beginning 5 seconds after the start of the spiral sequence. Image reconstruction and processing were performed off-line to yield images of relative regional cerebral blood volume (rCBV) (calculated from the integral of δR2* with respect to time) and time-to-peak (TTP) of bolus.
Three-dimensional time-of-flight MR angiography of the circle of Willis was performed with parameters of 53/4/1, a section thickness of 1.2 mm, a flip angle of 20°, an FOV of 22 × 15.5 cm, a matrix of 512 × 192, and two slabs, for a total of 52 sections.
All images were reviewed by two board-certified neuroradiologists and one MR physicist, all of whom are familiar with stroke imaging and, in particular, with reading diffusion-weighted and diffusion images. Images were interpreted at the time of the examination and retrospectively.
Case 1
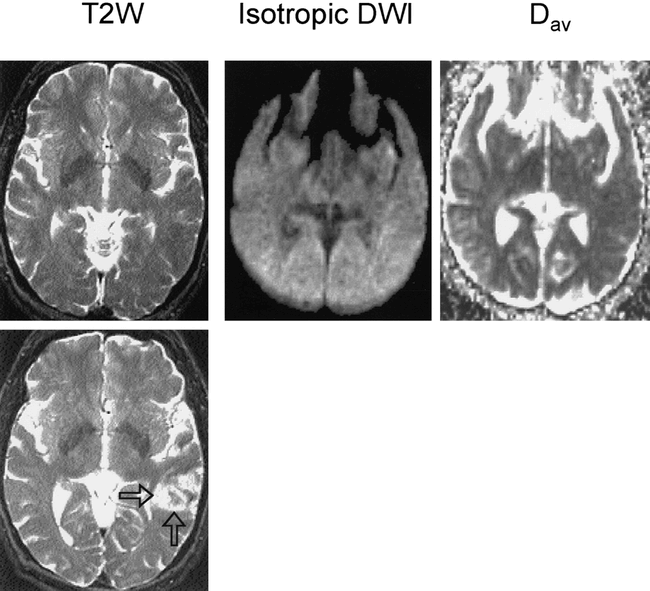
A 67-year-old man with a history of transient ischemic attacks and risk factors for cardioembolism (atrial fibrillation and myocardial infarctions) awoke with word-finding and comprehension difficulties. He had been off Coumadin for 4 days prior to pacemaker insertion and had experienced episodic left-sided amaurosis fugax. On examination, mild to moderate Wernicke aphasia and mild right arm apraxia were noted, with no motor or sensory deficits. The clinical diagnosis was acute embolic stroke in the left temporal region. He subsequently underwent an unremarkable CT examination, followed by MR imaging at 4 hours (Fig 1). Findings on conventional spin-echo T1- and T2-weighted images and on diffusion-weighted and Dav images were entirely normal. Measured diffusion constants (see Table) were normal and showed no left/right asymmetry. Perfusion imaging was not performed owing to the patient's refusal of contrast material.
Calculated diffusion constants (Dav) × 10−3 mm2/s (mean ± SD) in 5- × 5-mm regions of eventual infarction (as identified on follow-up MR images) and in contralateral hemisphere
Case 1: 67-year-old man with word-finding and comprehension difficulties imaged 4 hours (top row) and 4 days (bottom row) after symptom onset. The T2-weighted (T2W), diffusion-weighted (Isotropic DWI), and Dav images were all normal at 4 hours; but at 4 days, the T2-weighted image showed an area of infarction in the left parietal region (arrows).
The decision was made not to administer thrombolytic treatment, since the patient was outside the 3-hour treatment window and had mild deficits (NIH Stroke Scale [NIHSS] score = 3); he received heparin anticoagulation instead. A follow-up conventional MR examination, performed 4 days later without diffusion weighting, showed a 3-cm cortical infarction in the posterior left temporal lobe (Fig 1).
Case 2
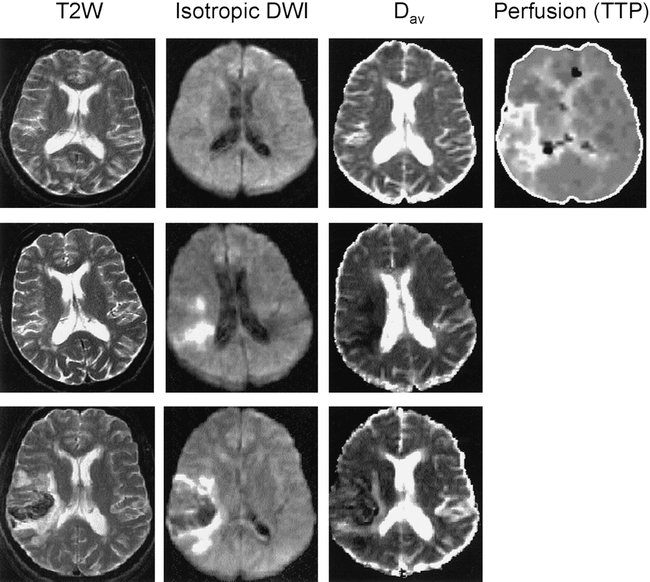
A 65-year-old man experienced sudden onset of word-finding difficulties and was found to have mild to moderate aphasia (expressive > receptive), a slight left-sided facial droop, and minimal left pronator drift on examination 2 hours after symptom onset. His symptoms were judged to be mild (NIHSS score = 3). His history was significant for diabetes mellitus, hypertension, and previous myocardial infarction, for which he received medical therapy. He was not considered a candidate for thrombolytic therapy because of the minimal deficits, and instead was treated with aspirin and blood pressure management. MR imaging was performed 3.5 hours after symptom onset and consisted of conventional T1- and T2-weighted images, diffusion-weighted images, and perfusion images. Findings on conventional images, diffusion-weighted images, and Dav images were entirely normal (Fig 2). Measured diffusion constants (Table) were normal and exhibited no left/right asymmetry at this time. Perfusion images (processed off-line and therefore unavailable immediately to influence patient management decisions) exhibited relatively normal rCBV, but a large region of markedly increased TTP was noted in the right middle cerebral artery (MCA) distribution, presumably reflecting decreased blood flow (Fig 2).
Case 2: 65 year-old man with mild to moderate aphasia, a slight left-sided facial droop, and minimal left pronator drift scanned 3.5 hours (top row), 13 hours (middle row), and 4 days (bottom row) after symptom onset.
Top row, Initial T2-weighted (T2W), diffusion-weighted (Isotropic DWI), and Dav images were normal, but the perfusion MR image revealed a large area of delayed flow in the right MCA territory.
Middle row, At 13 hours, the T2-weighted image remained normal but diffusion-weighted hyperintensity and reduced Dav are now apparent in the right MCA territory.
Bottom row, T2-weighted image at 4 days revealed hemorrhagic infarction with edema and mild mass effect. The infarct showed decreased Dav at this time, while the perilesional edema had increased Dav.
The patient deteriorated overnight, progressing to global aphasia and incurring a new left visual field neglect (NIHSS score = 11). A repeat MR examination was performed 13 hours after onset (conventional MR imaging, diffusion-weighted imaging, and additional MR angiography, but no perfusion imaging), at which time a diffusion abnormality in the right temporoparietal region was visible, whereas the T2-weighted images remained normal (Fig 2). The MR angiogram (not shown) exhibited markedly decreased flow signal in the right MCA. Hypervolemic therapy and heparin anticoagulation were initiated on the assumption this was an embolic event from a cardiac source. No acute response to this treatment was observed.
A follow-up MR study performed 4 days later revealed hemorrhagic transformation of the infarction with surrounding vasogenic edema and mild mass effect (Fig 2). The combination of T2-weighted, diffusion-weighted, and Dav images helped to distinguish regions of edema from those of infarction (on the basis of high Dav associated with edema). The patient's neurologic status continued to improve, until several days later, when newly decreased alertness and increased left-sided weakness were noted. A head CT scan showed increased mass effect resulting from the hemorrhagic infarction, and prompted the discontinuation of anticoagulation. The patient gradually improved, but at the time of transfer for occupational and speech therapy, 25 days after admission, he had a persistent severe expressive aphasia, mild to moderate left arm weakness, a continuing need for nasogastric tube feeding, and mild left-sided neglect.
Discussion
Both patients described here were symptomatic at the time of imaging but had normal findings on T2-weighted, diffusion-weighted, and Dav images at approximately 4 hours after onset of stroke. Patients subsequently progressed to infarction at sites initially showing normal diffusion. Initial Dav measured in the regions of later infarction in both patients were within normal limits and not significantly different from those in corresponding regions in the opposite hemisphere (see Table). In case 2, by 13 hours, hyperintensity was clearly visible on diffusion-weighted images and Dav was significantly decreased. Perfusion imaging performed in this patient at 4 hours showed delayed flow (increased TTP), corresponding to the site of later hemorrhagic infarction; however, the need for off-line reconstruction and processing to yield the perfusion maps meant the information was not immediately available for clinical decision making. These patients represent two (33%) of six patients studied less than 6 hours after stroke onset, and, including the third patient with negative findings on diffusion-weighted images (not shown), three (30%) of a small series of 10 patients studied less than 24 hours after ictus. All the other patients had at least some initial diffusion-weighted hyperintensity, and all were later found to have larger infarction volumes.
Imaging techniques that can lead to a positive diagnosis of acute stroke in humans are urgently required, as new methods of treatment become increasingly available. A positive diagnosis of stroke is important, since many of these therapies are expensive and also potentially harmful if applied to the wrong patient (7). Diffusion-weighted MR imaging is generally considered to be one of the most sensitive MR imaging techniques for detecting cerebral ischemia and infarction (1, 8). While diffusion-weighted imaging has been studied extensively in acute (1 to 2 days) stroke (8), relatively few patients have been studied within the 6-hour hyperacute period, believed to be the optimal window for treatment success, and very few studies have focused exclusively on this clinically critical period (3, 4, 9). Those investigators who have concentrated on this time period have generally reported diffusion-weighted imaging to be both highly sensitive (90% to 100%) and specific (100%) (3, 4, 9). Lövblad et al (4) recently reported that of 151 patients with eventual proved infarction studied within 24 hours of stroke onset, 18 had negative findings on diffusion-weighted images, and the negative predictive value (ie, the probability that a negative diffusion-weighted image would correspond to no subsequent infarction) was only 69.5%. It was noted, however, that these 18 negative diffusion-weighted findings were mostly the result of “lesions that were beyond the resolution of the scanner (minor or resolving deficits clinically localized to the brain stem)” (4). On the other hand, in both patients described in this report, the cortical infarcts were ultimately easily visible.
Region-of-interest measurements in our study showed the diffusion constant to be 4% to 9% lower in the eventual region of infarction than that measured in the contralateral hemisphere; however, this difference was not statistically significant. The clinically important issue is that these changes, if present, were too subtle to be identified by three independent readers at the time of the imaging study. It may also be argued that the lesions may have been visible at higher b-values. However, our maximum b-value (822 s/mm2) was actually higher than that used by Tong et al (741 s/mm2) (9) and slightly lower than that used by Lövblad et al (1000 s/mm2) (10). For the current cases, if we take the average ischemic Dav value as 0.77 mm2/s and the normal brain Dav value as 0.81 mm2/s, increasing the b-value from 822 to 1000 s/mm2 would change the diffusion-weighted contrast (defined as the signal difference between ischemic and normal signal, divided by the normal signal) from 3.3% to 4.1%, which is a negligible difference. Even at a b-value of 1500 s/mm2, contrast would only be 6.2%, which would be difficult to visualize given the decreased signal-to-noise ratio associated with higher b-values. In the other seven patients in this series, and in the follow-up images of case 2, a b-value of 822 s/mm2 was more than adequate to visualize diffusion-weighted hyperintensity in the stroke location.
We believe there are three potential mechanisms that may explain the lack of diffusion changes in the acute phase in these patients with proved eventual infarction. First, it is possible that cerebral blood flow (CBF) was at an intermediate level below the threshold for neuronal dysfunction (symptom onset) but above that of reduced diffusion. Hössman (11) has recently reviewed the literature regarding blood flow thresholds in ischemia (mainly in animal models); generally, suppression of EEG activity (corresponding to deficit onset) occurs when perfusion is in the range of 15 to 20 mL/100 g per minute (30% to 40% of normal CBF of 50 mL/100 g per minute) (12, 13), whereas membrane pump failure (which is the phenomenon associated with the bulk of the diffusion changes) (14) does not occur until below 10 to 15 mL/100 g per minute (15). Relatively few studies, however, have been done of blood flow thresholds for diffusion changes (16, 17), and comparing findings between species with different basal blood flows can be difficult. It may also be difficult to compare studies done with different diffusion-weighted imaging or CBF techniques. Moreover, studies of the development of dynamic infarction have found that these thresholds are not static but may increase with time, perhaps indicating increasing susceptibility to injury as ischemia persists (18). The second possible mechanism for the absence of diffusion changes (only in the case in which perfusion imaging and MR angiography were not performed) may be that reperfusion had occurred, restoring the diffusion constant to normal (19) but not preventing eventual delayed infarction (20). Third, it is possible that a second ischemic event may have caused the eventual infarction. This seems unlikely, however, except in those patients in whom significant neurologic deterioration occurred after the initial imaging. This was the situation for case 2, but we note that this patient had an initial perfusion deficit that matched the site of eventual infarction.
In both cases, the presenting symptoms were mostly limited to aphasia (NIHSS score = 3 or 4), suggesting relatively smaller ischemic regions possibly due to either branch occlusion or the presence of excellent collateral flow, as opposed to involvement of the entire MCA territory. Often, patients may ignore mild neurologic deficits but not aphasia, since this has a significant impact on normal life activities. This tendency may account for the relatively high proportion of patients presenting with aphasia but without other deficits in our series. In this respect, our patients may differ from the two patients with negative diffusion-weighted findings reported by Tong et al (9), who had much more severe presenting deficits (NIHSS score = 17 and 24, respectively).
Conclusion
These cases of normal diffusion-weighted imaging findings in the setting of impending infarction emphasize the need for timely perfusion assessment in hyperacute stroke patients, especially if thrombolytic therapy is contemplated. There is no question that diffusion-weighted imaging is an important diagnostic tool in stroke management, providing the means to confirm cerebral ischemia and infarction in many cases with higher sensitivity than that afforded by CT or conventional MR imaging (7, 8). In some patients, however, cerebral perfusion may be decreased without any associated diffusion abnormality.
Acknowledgments
We thank Jeff Duyn and Yihong Yang (NIH) for the spiral perfusion pulse sequence.
Footnotes
↵1 Supported in part by Established Investigator awards from the American Heart Association (P.B.B., P.C.M.v.Z.) and by NIH grant R01 NS31490 (P.C.M.v.Z.). N.J.B. is supported by a grant from the American Roentgen Ray Society.
2 Address reprint requests to Peter B. Barker, PhD, Department of Radiology, MRI 143C, Johns Hopkins University School of Medicine, 600 N Wolfe St, Baltimore, MD 21287.
References
- Received December 23, 1998.
- Accepted after revision March 9, 1999.
- Copyright © American Society of Neuroradiology