Abstract
Summary: We present two cases of hyperacute ischemic stroke that were initially missed by diffusion-weighted imaging; abnormalities in locations corresponding to focal neurologic deficits were discovered by MR angiography and perfusion-weighted imaging. Within hours, follow-up diffusion-weighted scans revealed partial conversion of the hypoperfused regions to complete stroke. These cases illustrate the potential for a nonresolving stroke-in-evolution to go undetected by diffusion-weighted imaging at hyperacute timepoints.
Diffusion-weighted imaging is an increasingly popular technique for the diagnosis of acute ischemic stroke. Because it reveals cytotoxic edema, an early histologic marker for developing infarction (1), it has been proposed as a superior approach to traditional methods such as CT for the examination of patients presenting with acute neurologic deficits. Animal studies reveal detectable changes on diffusion-weighted images within 1 hour of middle cerebral artery occlusion (2) and on apparent diffusion coefficient (ADC) maps within 3 minutes of global occlusion (3). In humans, the lower limits of ischemic duration for diffusion-weighted imaging detectability are not established, although given the histopathologic underpinnings of this technique, it seems reasonable to suggest that all ischemic strokes above the limits of spatial resolution will be detectable by the time the patient can be scanned. Supporting this view, a series of 24 patients with zero false-negative findings for acute stroke has been reported (4). Although reports can be found in abstract form, both supporting (5) and refuting (6–8) the unblemished sensitivity of diffusion-weighted imaging for hyperacute ischemic stroke, false-negative findings in the radiologic literature have generally consisted of resolving deficits (9–11) or radiographically unconfirmed strokes (10, 11). In the case reports that follow, we present two clinically and radiologically documented examples of patients progressing to complete stroke after an initial negative diffusion-weighted imaging finding.
Case Reports
Case 1
A 72-year-old white woman was transferred to our hospital for further management of unstable angina, with complaints of burning in the throat radiating to the left arm. The patient had reported similar symptoms for several days prior to admission, mostly occurring at rest. Acute myocardial infarction was ruled out by serial creatine kinases. Heparin was stopped at 4:00 a.m. on the fourth day after hospital admission, pending cardiac catheterization. At 6:50 a.m. the same day, the patient stated that she felt as though she had had a stroke. She complained of right arm weakness, throat discomfort, a sensation of tongue swelling, and noticeable difficulty with speech. Physical examination at that time was significant for new-onset right-arm flaccid paralysis and right lower extremity weakness. Additional findings included expressive aphasia, left gaze deviation bilaterally, and right gaze palsy. There was also a right facial droop with some loss of the nasolabial fold. Sensory examination was significant for decreased light touch and vibratory sensation in the right upper and lower extremities.
MR imaging was performed with a diffusion-weighted sequence (echo-planar; three orthogonal sets of diffusion sensitization; b = 1000; 6000/99 [TR/TE]; slice thickness, 7.0 mm; gap, 0; FOV, 240 mm; matrix, 96 × 200) performed at approximately 3 hours after symptom onset. Because of physiologic diffusional anisotropy in white matter tracts, hyperintensity on diffusion-weighted images was interpreted as representing cytotoxic edema and, therefore, acute ischemic stroke only if present in all three diffusion-sensitized directions. This is the equivalent of accepting hyperintensity on a single-axis diffusion-weighted image as positive for stroke if locations or configurations thought to represent normal anisotropy are not counted (12). Trace diffusion images, representing the average of three orthogonal sets of images sensitized to diffusion in a single direction, were not used because of image shifts in the phase direction uncorrected by the reconstruction software available at the time. With these criteria, no acute ischemic stroke was identified (one reader, D.M.L.) on diffusion-weighted imaging (Fig 1A). T2*-weighted (echo-planar free-induction decay) images were negative for hemorrhage. Perfusion-weighted imaging using a gadolinium-bolus tracking T2*-weighted (echo-planar free-induction decay) sequence generating time-to-peak maps with a temporal resolution of 2 seconds showed a large area of hyperintensity representing delayed flow in the superior aspect of the left middle cerebral artery territory (Fig 1B). Maximum-intensity projections from a three-dimensional time-of-flight (3D-TOF) MR angiography sequence revealed considerable irregularity and narrowing to both carotid siphons, and mildly decreased flow-related enhancement in the left internal carotid artery.
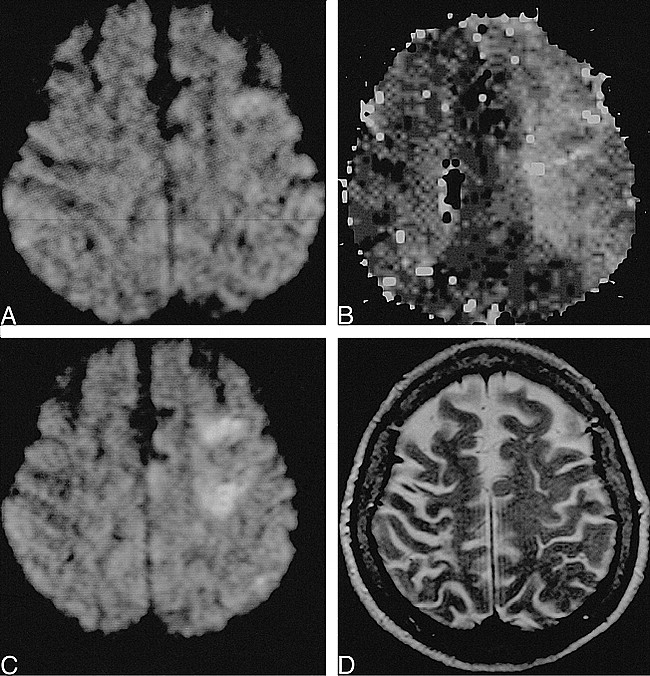
fig. 1. 72-year-old woman scanned 3 and 8 hours post ictus.
A, Diffusion-weighted imaging reveals a questionable area of restricted diffusion in the left frontal lobe, not identified prospectively.
B, Perfusion-weighted imaging demonstrates a large region of delayed time-to-peak (hyperintensity) in the left middle cerebral artery territory.
C, Diffusion-weighted imaging at 8 hours shows acute cortical and subcortical infarcts in the left frontal watershed zone.
D, T2-weighted image at 8 hours shows no abnormality.
Heparin was restarted. The patient's clinical status over the subsequent 5 hours showed increased somnolence, but no progression of focal deficits. Follow-up MR imaging at 8 hours post ictus now revealed acute cortical and subcortical infarcts on diffusion-weighted images in the anterior watershed zone of the left hemisphere (Fig 1C). These findings were not discernible by proton density–or T2-weighted imaging (Fig 1D). T2*-weighted images remained negative for hemorrhage. Perfusion-weighted imaging and MR angiography were not repeated.
Case 2
An 81-year-old white woman with a history of hypertension and coronary artery disease presented with acute aphasia, right facial droop, and right hemiparesis. At initial presentation, the patient was in atrial fibrillation. Neurologic examination revealed a flattened right nasolabial fold and decreased strength of right eye closure. The right upper and lower extremities were flaccid and without sensation. Babinski was upgoing on the right.
MR imaging was performed approximately 2 hours after symptom onset. No acute ischemic stroke or hemorrhage was identified by diffusion-weighted (Fig 2A) and T2*-weighted imaging, respectively. T2-weighted imaging revealed only a moderate number of nonspecific ischemic changes in the centrum semiovale bilaterally. Although postprocessing software for perfusion maps was not yet available, intensity versus time curves of the passage of a gadolinium-bolus showed an approximately 6-second delayed transit to distal left middle cerebral artery vessels (Fig 2B and C). Markedly decreased flow-related enhancement in the left middle cerebral artery territory was observed on a 3D-TOF sequence (Fig 2D).
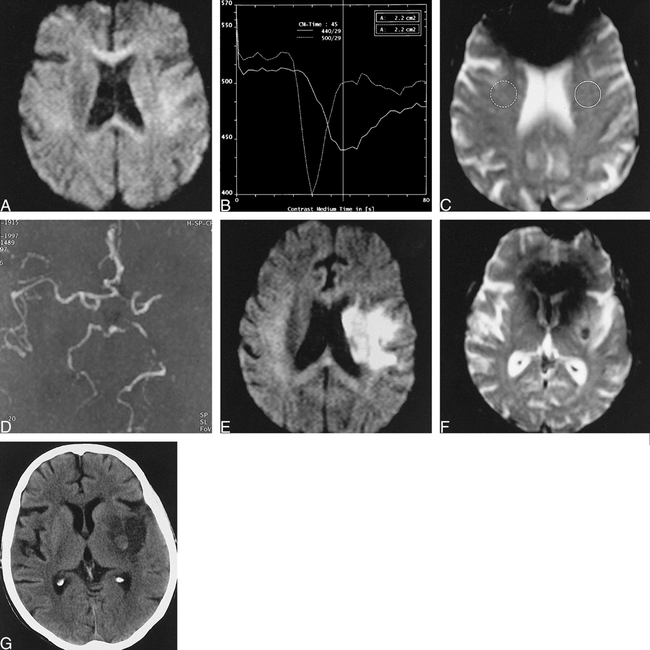
fig. 2. 81-year-old woman scanned 2 and 19 hours post ictus.
A, Diffusion-weighted imaging at 2 hours shows subtle restricted diffusion in the left deep cerebral structures, not appreciated prospectively.
B and C, Perfusion-weighted imaging bolus-tracking curves (B) for selected regions (C) show approximately 6-second delayed transit to the distal left middle cerebral artery vessels (solid line).
D, MR angiography demonstrates decreased flow-related enhancement in the left middle cerebral artery territory.
E, Diffusion-weighted imaging at 19 hours reveals acute infarct in left basal ganglia and insular cortex.
F, T2*-weighted imaging demonstrates a new 1-cm focus of profound hypointensity in the left putamen, consistent with focal hemorrhage or localized desaturation of hemoglobin.
G, CT scan at 2 days reveals a 1-cm focus of petechial hemorrhage in the left putamen surrounded by ischemic infarct.
Recombinant tissue plasminogen activator (r-tPA) was administered intravenously at just under 3 hours post ictus. Follow-up MR imaging at 19 hours post ictus now revealed an acute infarct in the deep cerebral structures on the left, shown to be extending to the insular cortex by diffusion-weighted imaging (Fig 2E) and shown to have vasogenic edema in these same areas by T2-weighted imaging. In addition, a new 1-cm focus of profound hypointensity on T2*-weighted images had developed in the left putamen, consistent with either focal hemorrhage or localized desaturation of hemoglobin (Fig 2F). MR angiography again showed poor flow-related enhancement distal to the left middle cerebral artery M1 segment. Perfusion-weighted imaging was not repeated.
The patient's course during the first 2 days post ictus included increased prominence of the right nasolabial fold and improved right-sided movement and pain response. Follow-up CT performed at 2 days revealed a new rounded 1-cm focus of petechial hemorrhage in the left putamen (Fig 2G) corresponding to the abnormality previously noted on T2*-weighted images. Moderate surrounding edema was noted, consistent with the acute middle cerebral artery stroke previously demonstrated on MR images.
Discussion
The sensitivity of diffusion-weighted imaging for hyperacute ischemic stroke depends on the standard of reference employed for diagnosis. Investigating the predictive value of diffusion-weighted imaging for clinical outcome, Warach et al (10) reported one false-negative finding out of 19 cases; however, no new stroke was documented on follow-up imaging. Sorensen et al (9) found two of 11 hyperacute stroke patients scanned within 10 hours post ictus showed negative findings on diffusion-weighted images; however, their symptoms resolved within 1 to 48 hours. Most recently, Lovblad et al (11) reported a diffusion-weighted imaging stroke sensitivity of 88%, which increased to 94% when only those patients scanned within 6 hours of symptom onset were included. The false-negative findings were either in patients with a final clinical diagnosis of stroke that were unconfirmed by imaging studies and therefore were attributed to small lesions below the limits of scanning resolution, or in patients who had resolving neurologic deficits. What remains unresolved by these studies is the sensitivity of diffusion-weighted imaging to unequivocal strokes, those confirmed by unambiguous tissue damage and fixed neurologic deficits.
The phenomenon of mismatches between diffusion-weighted and perfusion-weighted imaging parameters is also well recognized (9–11). Typically, regions of hypoperfusion extend beyond the zone of restricted diffusion, and have been proposed to represent an MR imaging equivalent of the ischemic penumbra (13). This observation is also taken as support for the concept of thresholds of ischemia (14) for various points along the clinical-histopathologic cascade, from presentation with neurologic deficits to completed infarction. Thus, hypoperfusion without restricted diffusion may produce symptoms; however, infarction may be limited to the zone demarcated by hyperintensity on diffusion-weighted images. The problem that this analysis poses for a purported 100% sensitivity to infarction of diffusion-weighted imaging is that it ignores the effect of extrapolating back to smaller and smaller diffusion-weighted imaging lesions if the size of the penumbra were to remain constant. At this end of the spectrum, an acute neurologic deficit should theoretically be associated with a perfusion-weighted imaging lesion only, because the blood flow decrement would not have been of sufficient magnitude or duration to cause cytotoxic edema. Should such an event progress to infarction, it would constitute a false-negative diffusion-weighted imaging result.
The cases reported herein appear to be representative of such a category. Both patients were scanned exceptionally early after symptom onset (3 and 2 hours post ictus, respectively), which evidently were insufficient durations for the development of cytotoxic edema at their degrees of hypoperfusion. It is instructive to note that both of these early false-negative scans occurred within the therapeutic window for thrombolysis with intravenous r-tPA. Unfortunately, hopes that diffusion-weighted images obtained within this time frame will provide an antidote to CT's relative insensitivity for hyperacute ischemic stroke detection (15) are at least partially dimmed by these cases. A larger series of patients who are scanned during such an early postictal period would be of great interest; however, one of the challenges in conducting such a study is the logistical difficulty of rapidly obtaining such scans to avoid jeopardizing treatment options.
Retrospective analysis of the initial scans in these patients included calculation of ADC ratios from region-of-interest measurements of the trace of the diffusion tensor in ischemic tissue to contralateral normal brain. Two small ischemic foci that ultimately developed in patient 1 had ADC ratios of 0.81 and 0.74 on the initial scans that progressed to 0.42 and 0.49, respectively, on follow-up scans. The center of an infarct in patient 2 that later became large had an ADC ratio of 0.75 on the initial scan and a ratio of 0.44 on the follow-up scan. Reinspection of the initial scans reveals subtle areas of increased signal intensity on diffusion-weighted images in some of the regions that subsequently progressed to infarction. The routine interpretation of such subtle areas as ischemic stroke, however, would probably result in many false-positive scans. Because of unacceptable blurring on our trace (combined) diffusion-weighted images, it was necessary for us to use three orthogonal diffusion-weighted sets with unidirectional diffusion sensitization. In so doing, the physiologic anisotropic diffusion of white matter may have posed a visual distraction, thus undermining the conspicuity of any potential lesion. This was probably the source of “error” in case 2, as the retrospectively identified hyperintensity is not much more than asymmetrical diffusional anisotropy. Further, when interpreting orthogonal diffusion-weighted imaging sets, there is a tendency to disregard subtle abnormalities that could be the result of magnetic susceptibility artifact, such as hyperintensity just below the left frontal bone, as in case 1. Nevertheless, despite these potential pitfalls, the use of orthogonal diffusion-weighted imaging sets for stroke diagnosis appears to have been validated in a recent study by Chong et al (12), who found when comparing four different methods of processing data from diffusion-sensitized scans, that orthogonal sets of images were more accurate than images combined in various ways. Regardless of the particular method employed, at the limits of diffusion-weighted imaging's hyperacute stroke sensitivity, our observations suggest that, if used in conjunction with MR angiography and perfusion-weighted imaging, ADC maps may help resolve ambiguous findings and thereby improve the accuracy of diffusion-weighted imaging.
Conclusion
Failure of diffusion-weighted imaging to reveal ischemic stroke prospectively in 2 patients within 3 hours of symptom onset supports the contention that additional techniques may be necessary at hyperacute time points to identify tissues at risk for infarction more thoroughly.
Footnotes
↵1 Presented at the 36th annual meeting of the American Society of Neuroradiology; May 1998; Philadelphia, PA.
Address reprint requests to David Lefkowitz, MD, Department of Diagnostic Radiology, University of Maryland Medical System, 22 S Greene Street, Baltimore, MD 21201-1595.
References
- Received January 15, 1999.
- Accepted after revision May 4, 1999.
- Copyright © American Society of Neuroradiology