Abstract
BACKGROUND AND PURPOSE: Endovascular recanalization has been attempted in patients with symptomatic chronic ICA occlusion, however, the heterogeneity of recanalization outcomes and the perioperative complications present challenges for the clinical application. Our aim was to evaluate the safety and efficacy of endovascular recanalization for symptomatic chronic ICA occlusion and identify potential predictors for successful recanalization.
MATERIALS AND METHODS: This study included 47 consecutive patients with symptomatic chronic ICA occlusion who underwent endovascular recanalization at our institution. Patients’ clinical information, radiologic characteristics, procedural results, and outcomes were recorded. Factors related to technical success were analyzed by univariate and multivariate analyses.
RESULTS: The technical success rate was 74.5% (35/47); 12.8% of patients (6/47) experienced intraoperative complications, but none had permanent neurologic deficits. Three months after recanalization, 21 of the 29 recanalized patients (72.4%) and 3 of the 10 failed patients (30.0%) demonstrated improved mRS scores. Restenosis or re-occlusion occurred in 12.9% of patients (4/31) with successful recanalization. Multivariate analysis showed that tapered or blunt stump (P = .016), distal ICA occlusion segment (below the cavernous segment versus at or above the ophthalmic segment, P = .003; at the cavernous or clinoid segment versus at or above the ophthalmic segment, P = .027), and radiologic occlusion to recanalization of ≤3 months (P = .044) were significantly associated with successful recanalization. Patients were assigned points according to the coefficients of the prediction model, and the technical success rates were 0%, 46.2%, 90.5%, and 100% in patients with 1, 2, 3, and 4 points, respectively.
CONCLUSIONS: Endovascular recanalization is a safe and effective treatment for symptomatic chronic ICA occlusion in selected patients. A residual stump, low levels of the distal ICA occlusion segment, and a short radiologic occlusion time were identified as positive predictors of technical success.
ABBREVIATIONS:
- AUC
- area under the curve
- CICAO
- chronic ICA occlusion
- HR-MRI
- high-resolution MR imaging
Most patients with chronic internal carotid artery occlusion (CICAO) can be treated successfully with aggressive medical management. However, some patients (6%–20%) remain at risk of recurrent TIA or ipsilateral ischemic stroke,1,2 which are mainly caused by impaired cerebral perfusion or a carotid stump embolism in the downstream circulation.3,4
The optimal treatment for symptomatic CICAO is unclear, especially in patients refractory to aggressive medical management. According to previous research, surgical revascularization with extracranial–intracranial artery bypass is not superior to medical therapy in patients with hemodynamic impairment and does not reduce the risk of recurrent cerebrovascular events.5,6 Some studies have demonstrated that carotid endarterectomy can successfully revascularize a short extracranial ICA occlusion but is ineffective for long segmental and intracranial lesions.7,8 Hybrid surgery seems to compensate for these drawbacks, but it is associated with increased perioperative stroke and mortality rates because of the complicated postoperative management related to open surgery and intensive antiplatelet or anticoagulant protocols.9 In recent years, some case series on endovascular recanalization monotherapy have been published. However, the procedure is technically demanding, has varying success rates (ranging from 53.3% to 93.3%), and carries a high risk of perioperative complications, such as dissection, distal embolization, and hyperperfusion syndrome.10,11 Moreover, research is limited by the small number of cases; therefore, the preoperative radiologic predictors of successful recanalization for symptomatic CICAO have not been thoroughly investigated.
Hence, we performed a retrospective study to evaluate the success rate and outcomes of endovascular recanalization for symptomatic CICAO at our center. Furthermore, we identified potential radiologic predictors of successful recanalization, which will help clinicians select appropriate candidates for this procedure.
MATERIALS AND METHODS
Study Population
This study retrospectively analyzed 47 patients with symptomatic CICAO who underwent endovascular recanalization at our institution from January 2018 to December 2021. All treatment choices were made according to the multidisciplinary consultation of the doctors in the neurology, neurosurgery, vascular surgery, and interventional radiology departments, along with the treatment wishes of patients and families. Chronic occlusion was considered as ≥4 weeks after onset. The inclusion criteria were as follows: 1) atherosclerotic CICAO, defined as 100% cross-sectional truncation of the vessel lumen observed by CTA or MRA and confirmed by DSA, 2) aggravation or recurrence of neurologic symptoms (TIA or stroke) despite aggressive medical treatment, and 3) hypoperfusion in the CICAO territory confirmed by preoperative CTP or MR perfusion imaging. The following exclusion criteria were applied: 1) acute occlusion of the carotid artery; 2) asymptomatic lesions; 3) nonatherosclerotic or dissection occlusion, including Moyamoya disease, vasculitis, and trauma; 4) neurologic symptom aggravation or recurrence due to hemorrhagic transformation of infarction, a new infarction in the nonoccluded vessel territory, hypovolemia or systemic hypotension, heart and kidney insufficiency, severe infection, or high fever; 5) occurrence of a bleeding disorder in the past 3 months; and 6) allergies or contraindications to contrast media, heparin, or anesthesia. This study was approved by the ethics committee of our institution and conducted in accordance with the mandates of the Declaration of Helsinki (2008). Because of the retrospective nature of the study, the requirement for patients to provide informed consent was waived.
Clinical and Radiologic Assessment
Patients’ clinical information, including the mRS score, was recorded. Two independent neuroradiologists performed all image assessments, and discrepancies were resolved by consensus. Brain CTP or MR perfusion was performed to evaluate cerebral hemodynamics and perfusion–diffusion mismatch. Cerebral hypoperfusion ipsilateral to the ICA occlusion was defined as delayed perfusion throughout the ipsilateral hemisphere with delayed TTP, increased MTT, and decreased regional CBF.12 High-resolution MR imaging (HR-MRI) was used to analyze the occlusion etiology, proximal stump morphology, distal ICA occlusion segment, occlusion length, and luminal thrombosis. Several patients had poor-quality HR-MRI or did not have HR-MRI; these patients underwent preoperative vascular assessment by multiple simulated CTA images, which were reconstructed from the CTP images. Occlusion length was automatically calculated by the software after manual tracing of the longitudinal axis of the occluded vasculature.13 Segments of the ICA were evaluated according to the classification criteria proposed by Bouthillier et al.14
Endovascular Treatment
Patients received aspirin, 100 mg/day, and clopidogrel, 75 mg/day, for at least 5 days before the procedure; subsequently, a thromboelastogram was used to evaluate platelet reactivity. Aspirin resistance was defined as <50% inhibition of arachidonic acid–induced platelet aggregation, and clopidogrel resistance was defined as <30% inhibition of adenosine diphosphate–induced platelet aggregation. No cases of aspirin resistance were observed, whereas 4 patients showed clopidogrel resistance and were treated with ticagrelor, 90 mg twice a day. All angiographic procedures and angioplasties were performed with the patient under general anesthesia by experienced neurointerventionalists. Intravenous heparin boluses were administered to maintain an activated clotting time of 250–300 seconds during the procedures.
Femoral access was achieved with an 8F sheath introducer and an 8F guiding catheter (Envoy, Codman Neuro) placed into the common carotid artery proximal to the occluded segment. Using roadmap guidance, the surgeon first attempted to pass a 0.035-inch Radifocus Guide Wire M (Terumo) in combination with a 4F vertebral angiographic catheter (Cordis) through the tapered stump or vulnerable area to the distal cervical ICA. Then, a 300-cm 0.014-inch exchange microwire (Transend or Synchro, Stryker) and an Echelon microcatheter (Medtronic) were used to advance further into the occluded segment and were navigated into the intracranial ICA. Thereafter, angiographic projections were performed to confirm the position of the microcatheter in the true lumen, assess the extent of the ICA thrombus, and confirm the patency of intracranial vessels. A SpiderFX embolic protection device (Medtronic) was typically used if short occlusions without tandem lesions and an adequate landing zone were identified. The Mo.Ma occlusion system (Medtronic) was sporadically used to protect against embolization in cases with suspected unstable thrombosis.
On the preoperative HR-MRI, high intraluminal signals on the unenhanced T1-sampling perfection with application-optimized contrasts by using different flip angle evolution (SPACE) sequence (Siemens) beyond the cervical occlusion site might have suggested intraluminal unstable thrombosis, and the procedure was commonly initiated by direct aspiration first. Otherwise, the procedure was initiated by predilation through the occlusive section with a small-diameter angioplasty balloon (2–3 mm), followed by closed-cell stent insertion, if appropriate. An open-cell stent was deployed for extremely tortuous vascular structures. A Wallstent (Boston Scientific), Precise stent (Cordis), or Protégé stent (Medtronic) was deployed for cervical ICAs. An Enterprise stent (Codman), LEO Plus stent (Balt), or Neuroform EZ stent (Stryker) was deployed for distal lesions.
If ≥2 stents were needed, they were deployed from the distal to the proximal region. The stent size was selected according to the proximal and distal diameters of the lesion and the length of the occluded segment. Postoperative angiography was performed to confirm patency and assess residual stenosis and distal perfusion. Post-balloon dilation was performed if residual stenosis within the stent was >50%. Successful recanalization was considered when the occlusion segment was stented with a final residual diameter stenosis of <30% and TICI grade 3 antegrade flow was established after intervention.
After the procedure, brain CT was performed immediately to rule out potential intracranial hemorrhage. Systolic blood pressure was strictly maintained at 100–120 mm Hg or decreased by 20% of the baseline value to prevent hyperperfusion syndrome. Regular dual-antiplatelet agents (aspirin, 100 mg/day, and clopidogrel, 75 mg/day), proper control of risk factors, and effective rehabilitation training were also prescribed.
Follow-up Outcomes
After discharge, dual-antiplatelet therapy was maintained for 3 months, and life-long aspirin or clopidogrel monotherapy was continued thereafter. Patients were also treated with statins and other medications to control risk factors. CTA or DSA was routinely performed 3 months after successful recanalization procedures and annually thereafter or when restenosis was suspected. Restenosis and re-occlusion were defined as a stenosis diameter of >50% and total occlusion of the target artery segment, respectively. Follow-ups were completed by clinic visits or telephone every 3 months until the end of April 2022. New neurologic deficits (TIA or stroke), intracranial hemorrhages, and deaths were recorded.
Statistical Analysis
All statistical analyses were performed with SPSS 25.0 (IBM). Categoric data are presented as counts and percentages. Continuous data are expressed as the mean (SD) or as the median and interquartile range. Univariate analysis was performed by the Fisher exact or χ2 tests for categoric data and 2-sample t tests or Mann-Whitney U tests for continuous data. To identify the factors independently associated with successful recanalization, we performed multivariable logistic regression; all variables with a P value of <.2 in the univariate analysis were included. ORs and their 95% confidence intervals were calculated. Then, a score-based prediction model was constructed with a regression coefficient–based scoring method. The points assigned to each variable were proportional to the regression coefficients of the variable rounded to the nearest integers. Statistical significance was set at a 2-sided P value of < .05.
RESULTS
Baseline Characteristics
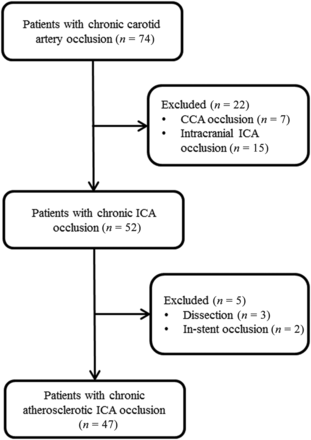
A total of 47 patients (42 men; mean age, 63.6 [SD, 9.6] years) with symptomatic CICAO were enrolled (Fig 1). Many patients had chronic comorbidities such as hypertension (66.0%), diabetes mellitus (34.0%), hyperlipidemia (10.6%), and cardiac disease (14.9%). In addition, 23 (48.9%) had a history of smoking, and 15 (31.9%) had a history of drinking. Preoperative vascular assessment by HR-MRI was performed in 38 patients (80.9%), and 9 patients (19.1%) were assessed by simulated multiple CTA images, which were reconstructed from the CTP images. The detailed patient characteristics and angiographic findings are presented in Table 1.
Patient flow diagram. CCA indicates common carotid artery.
Baseline characteristics of the studied patient population as stratified by failed or successful recanalization
Procedural Outcomes and Follow-up
A total of 35 (74.5%) patients achieved successful recanalization. After the wires crossed the occlusion, embolic-protection devices were used in 34.3% (12/35) of patients: Ten patients received the SpiderFX device and 2 received the Mo.Ma occlusion system. The overall intraoperative complication rate was 12.8% (6/47); 1 patient had a slight subarachnoid hemorrhage followed by microwire perforation, 3 patients had asymptomatic dissection (2 of whom were treated with stent implantation), and 2 patients had distal embolization. Neither of the latter 2 patients exhibited new neurologic symptoms with successful mechanical thrombectomy. The rate of stroke or death within 30 days was 6.4% (3/47). One older adult patient died of massive reperfusion hemorrhage 1 day after the procedure. The other 2 patients developed new neurologic symptoms 2 and 10 days after their procedures (their mRS scores were 2 and 4, respectively). CTP revealed significant hypoperfusion on the recanalization side, and CTA showed in-stent thrombosis. The symptoms of both patients improved after emergency mechanical thrombectomy (local arterial thrombolysis combined with balloon dilation) and subsequent drug and rehabilitation treatment. No strokes or deaths occurred during hospitalization in the failed recanalization group.
The median follow-up period was 13.0 months (range, 2.3–51.8 months). At the 3-month follow-up, 21 of 29 patients having undergone recanalization (72.4%) with preoperative mRS scores of ≥1 showed improvement in their mRS scores, whereas only 3 of 10 patients (30.0%) with failed recanalization and preoperative mRS scores of ≥1 showed improvement in their mRS scores. Restenosis or re-occlusion occurred in 12.9% of recanalized patients (4/31) who had follow-up imaging: Three presented with asymptomatic re-occlusion, and the other presented with symptomatic restenosis (recurrent dizziness). The latter patient received subsequent balloon dilation therapy. No recurrent TIA or stroke occurred in the other patients with successful recanalization during the clinical follow-up period. Of the 12 patients with failed recanalization, 3 patients (25.0%) experienced recurrent TIA or stroke and 1 patient with stroke died despite aggressive medical management.
Predictive Factors and Construction of a Scoring System
No significant differences were found in demographics, risk factors, or symptoms between the successful and failed recanalization groups. The distal ICA occlusion segment tended to be at a lower level in the successful recanalization group than in the failed recanalization group (technical success rates: below the cavernous segment, 94.1%; at the cavernous or clinoid segment, 76.2%; at or above the ophthalmic segment, 33.3%; P = .004). Multivariate analysis with logistic regression revealed that the following factors were significantly associated with successful recanalization: tapered or blunt stump (OR = 25.8; 95% CI, 1.8–364.7; P = .016), distal ICA occlusion segment (below the cavernous segment versus at or above the ophthalmic segment) (OR = 306.6; 95% CI, 6.6–14,168.9; P = .003); at the cavernous or clinoid segment versus at or above the ophthalmic segment (OR = 11.4; 95% CI, 1.3–98.0; P = .027), and radiologic occlusion to recanalization of ≤3 months (OR = 10.9; 95% CI, 1.1–111.5; P = .044).
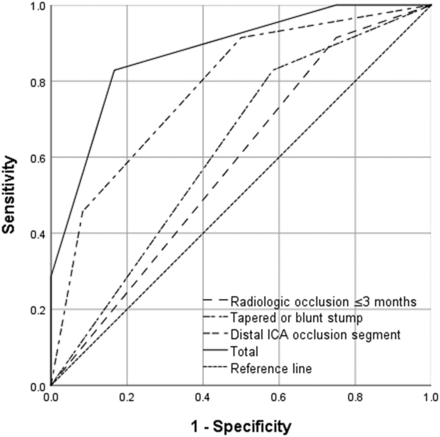
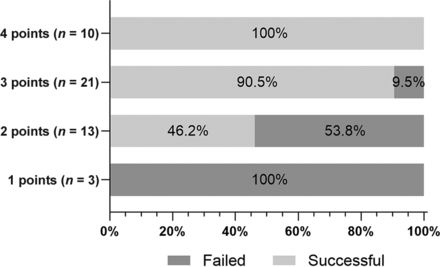
By means of the independent predictors obtained from multivariate analysis, a scoring system was created to predict the technical success rate of CICAO recanalization. Points for each variable were assigned as described in the materials and methods section (Table 2). Receiver operating characteristic curves were plotted to evaluate the predictive value of each factor and the combination of the 3 factors (Fig 2). The results demonstrated that the combination of the 3 factors had the highest efficacy for predicting successful recanalization (area under the curve [AUC] = 0.876). Furthermore, the technical success rates were 0%, 46.2%, 90.5%, and 100% in patients who were assigned 1, 2, 3, and 4 points, respectively (Fig 3). A representative case is presented in Fig 4.
ROC curves were plotted to evaluate the predictive value of every factor (a tapered or blunt stump, the level of distal ICA occlusion segment, radiologic occlusion to recanalization of ≤3 months) and the combination of the 3 factors. The results show that the combination of these 3 factors showed the highest efficacy (AUC = 0.876). ROC indicates receiver operating characteristic.
Effects of the presence of 1–3 predictive radiologic factors associated with failed or successful recanalization. The y-axis shows each group of patients with scores of 1, 2, 3, and 4 points; the actual number of patients is marked. The x-axis shows the percentage of patients who failed or succeeded in recanalization in each group. The technical success rates were 0%, 46.2%, 90.5%, and 100% in patients who were assigned 1, 2, 3, and 4 points, respectively.
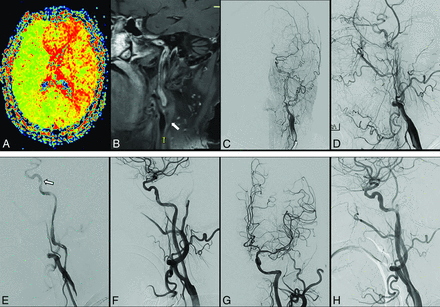
An elderly man presented with recurrent numbness and weakness in the right limb and was diagnosed with left ICA occlusion for >9 months. Preoperative perfusion-weighted imaging shows large areas of hypoperfusion (increased TTP, A) in the left hemisphere. HR-MRI scans (B) and angiographic results (C and D) confirm the occlusion of the C1 segment with no stump (arrow). The patient was assigned a score of 2 points before recanalization. After the microwire passed the occlusion site and was predilated with a 3 × 30 mm balloon, angiography (E) shows a long, rough vascular wall and regional dissection (arrow). Successful recanalization with good antegrade perfusion was achieved followed by the insertion of 1 Neuroform EZ stent (4.5 × 30mm) and 2 Wallstent stents (7 × 40 mm and 9 × 30 mm, F and G). Angiographic results show patent stents at the 3-month follow-up (H).
Multivariate analysis of factors influencing the successful recanalizationa
DISCUSSION
Recently, several studies with small sample sizes have reported that endovascular recanalization is a feasible strategy to treat symptomatic CICAO. However, the heterogeneity of recanalization outcomes and the perioperative complications presents challenges for the clinical application of endovascular recanalization.10,11 Our study observed an overall success rate of endovascular recanalization of 74.5% (35/47); the intraoperative complication rate was 12.8%, and the morbidity and mortality rate within 30 days was 6.4%. Furthermore, CICAO with stump (tapered or blunt), low levels of the distal ICA occlusion segment, and radiologic occlusion to recanalization of ≤3 months were associated with a greater chance of successful recanalization.
In previous studies, successful recanalization has been achieved in 53.3%–93.3% of patients with CICAO; this rate is related to the technical experience of the operators and the radiologic characteristics of the occlusion.10,11,15⇓-17 Our study, which focused on symptomatic atherosclerotic cases, found that a tapered or blunt stump had a greater chance of successful recanalization compared with no stump; this finding is consistent with those of previous studies.15,16 In addition, we found that a longer duration of the occlusion was associated with a lower probability of successful recanalization: Technical success decreased from 78.0% to 50.0% when the radiologic occlusion time was increased from ≤3 months to >3 months; the multivariate analysis also indicated that the occlusion duration played a significant role in recanalization. Similar results were found in a meta-analysis by Cagnazzo et al,11 which reported a successful endovascular recanalization rate of 70.2% for an occlusion duration of ≤3 months compared with 51.4% for an occlusion duration of >3 months.
The length of the occlusion is a major determinant of successful recanalization due to the level of difficulty in identifying the vascular course and deviation from the vessel lumen when crossing a long occlusion.17 In previous studies, the length of the occlusion was mainly judged according to the distance between the site of the proximal occlusion and the retrograde reconstruction of the distal ICA segment by the collateral vessels on delayed angiographic images, which might be longer than the true length of the underlying thrombotic occluded lesion.16,18,19 HR-MRI can be used preoperatively to directly visualize and provide detailed information about occluded segments.20 In the present study, we mainly classified the level of the distal ICA occlusion segment (Bouthillier segmentation) according to the preoperative HR-MRI and confirmed it by intraoperative angiograms; theoretically, this strategy is more accurate than the method of using delayed angiographic images. We found that low levels of the distal ICA occlusion segment were significantly associated with successful recanalization. Similar results were reported by Chao et al, who observed a higher technical success rate among “short-type” occlusions (occlusion length of ≤3 segments) compared with “long-type” occlusions (93.3% versus 57.8%).15 Most surprising, we found that an occlusion length of ≤5 cm was not significantly associated with successful recanalization. A study by Hasan et al18 reported a 100% success rate in patients with an occlusion length of <5 cm in the cervical ICA, whereas the success rate was 50% for longer lesions. This outcome could be ascribed to several factors: first, carotid artery segment lengths are heterogeneous because of differences in sex, age, and other factors; second, the carotid artery is tortuous upward. Thus, neither DSA nor HR-MRI could accurately measure the length of the occlusion.
Chen et al16 developed a scoring system to predict the success rate of endovascular recanalization; the system was based on angiographic features and symptoms, such as a nontapered stump, distal carotid artery reconstitution at the communicating or ophthalmic segments, and the absence of neurologic events. However, their study was limited by heterogeneity and bias; 43.5% of patients were asymptomatic, and patients with intracranial occlusion and nonatherosclerotic or dissection occlusion were also included. Using the data obtained, we constructed an easy-to-operate 3-factor model to predict successful recanalization preoperatively. The 3 factors in the model were a tapered or blunt stump, a distal ICA occlusion segment, and radiologic occlusion to recanalization of ≤3 months; these factors separately reflect CICAO features in the different aspects discussed above. In the 47 patients included in our study, the model achieved high efficacy in risk stratification. Patients who received 1 point in this scoring system achieved no success in recanalization surgery (0/3), whereas all patients who received 4 points achieved successful recanalization (10/10).
Complications after recanalization have been reported in close to 18% of patients, with higher rates (approximately 25%) among patients with longer occlusions.18,21 In our study, the rate of intraoperative complications (such as dissection and perforation) was 12.8%, with no permanent neurologic deficits remaining after the operation. Distal embolization is a common complication of recanalization for CICAO, with reported rates of up to 7.1%.15,18,19 In our study, 2 patients (2/47, 4.3%) demonstrated thrombus migration during the procedure; fortunately, no new symptoms or signs were observed after successful mechanical thrombectomy. Research has shown that atherosclerotic CICAO is mainly caused by the superposition of thrombosis due to stenosis. Disrupting the thrombus by microcatheter or microguidewire manipulation or compressing it during balloon dilation leads to a risk of thrombus migration.22 To stabilize the stenotic lesion and prevent distal embolization, we typically used a small-diameter balloon (2–3 mm) to perform predilation through the occlusive section first, followed by closed-cell stent insertion, if appropriate. Nevertheless, embolic protection measures should be performed to prevent embolism during recanalization procedures. However, the deployment of distal embolic protection devices, such as SpiderFX, is difficult when passing through the target vessels because of the long fibrotic or thrombosis lesion and tortuous vascular anatomy that is often found in chronic total occlusion.15,18,19 This issue is evidenced by the low application rate (34.3%, 12/35) of embolic protection devices in our study. The application of proximal protection devices that work through flow reversal, such as the Mo.Ma occlusion system, may be a beneficial supplementary technique to prevent distal embolization;23 however, studies with large sample sizes are needed to confirm the results in patients with CICAO.
The successful recanalization of CICAO leads to the resolution of the penumbra and the normalization of TTP or MTT, and it eventually contributes to the improvement of mRS scores following the procedure.12 In 2 other studies on endovascular therapy,24,25 significant improvements in neurologic deficits and cognitive function were observed 3–6 months after the recanalization procedure. Similarly, in our study, most patients (72.4%) with successful recanalization showed improvement in their mRS scores at the 3-month follow-up compared with baseline, whereas few patients (30%) with failed recanalization showed improvement in the mRS scores. One recanalized patient (1/34, 2.9%) developed recurrent dizziness due to in-stent restenosis, whereas 3 patients with failed recanalization (3/12, 25.0%) experienced recurrent TIA or stroke during follow-up. Although we could not use preoperative and postoperative mRS scores alone to measure the operative success of this intervention, the combination of the improvement in mRS scores associated with successful recanalization and the reduction in recurrent cerebrovascular events demonstrates the safety and efficacy of this procedure.
The present study has several limitations. First, it is a retrospective study with a modest sample size, which might interfere with the statistical analysis, and it may have contained correlative variables. Therefore, further prospective studies with large sample sizes are needed to verify the results. Second, the lack of follow-up imaging data for some patients might limit the evaluation of the overall restenosis/re-occlusion rate. Finally, few studies have focused on the mural and intraluminal thrombus signals on HR-MRI; thus, further studies are needed to identify potential predictors of successful recanalization.
CONCLUSIONS
For symptomatic CICAO, endovascular recanalization is safe and effective in selected patients and may improve neurologic symptoms while reducing the recurrence rate of TIA or stroke in the midterm. A tapered or blunt stump, low levels of the distal ICA occlusion segment, and a short radiologic occlusion time were identified as independent predictors of technical success. Although additional prospective randomized trials with large cohorts are necessary to confirm our results, the scoring system we constructed can help facilitate and improve preoperative case selection for recanalization.
Footnotes
C. Zhou, Y.Z. Cao, and S. Liu contributed equally to this work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received August 31, 2022.
- Accepted after revision January 24, 2023.
- © 2023 by American Journal of Neuroradiology