Abstract
BACKGROUND AND PURPOSE: Antithrombotic therapy following carotid artery stent placement with concomitant atrial fibrillation is not well-established. Our aim was to assess the safety and efficacy of the combination of direct oral anticoagulants and a P2Y12 inhibitor at 30 days after carotid artery stent placement in patients with atrial fibrillation.
MATERIALS AND METHODS: We designed an observational single-center study including patients who underwent carotid artery stent placement with concomitant atrial fibrillation. We studied 3 groups according to antithrombotic therapy: 1) the direct oral anticoagulants plus clopidogrel (DC) group: receiving direct oral anticoagulants plus a P2Y12 inhibitor; 2) the triple therapy group: anticoagulation and dual antiplatelet therapy; and 3) the dual antiplatelet therapy group: following dual antiplatelet therapy alone. The safety outcome was a major or clinically relevant non-major bleeding event at the first month. The efficacy outcomes were the thromboembolic events (myocardial infarction, stroke, systemic embolism, or stent thrombosis).
RESULTS: Of 959 patients with carotid artery stent placement, 91 met the inclusion criteria, including 24 patients in the DC group, 42 patients in the triple therapy group, and 25 in the dual antiplatelet therapy group. The mean age was 72.27 (SD, 8.1 ) years, with similar baseline characteristics. The median CHA2DS2-VASc score for each group was 6 (interquartile range = 5–6), 5 (interquartile range = 4–6), and 5 (interquartile range = 4–6), respectively. The median HAS-BLED score was 4 in the 3 groups (P = .17). The primary safety end point was 23.8% in the triple therapy group compared with 4% in the dual antiplatelet therapy group (P = .032), with no bleeding events in the DC group (P = .007). There was 1 stent thrombosis in DC group and a cardioembolic stroke in the dual antiplatelet therapy group (P = .41).
CONCLUSIONS: Among patients with carotid artery stent placement with atrial fibrillation, triple therapy confers a high bleeding risk. A regimen of direct oral anticoagulants plus a P2Y12 inhibitor might confer a good safety profile with significantly lower rates of bleeding and optimal efficacy.
ABBREVIATIONS:
- AF
- atrial fibrillation
- CAS
- carotid artery stent placement
- CS
- carotid stenosis
- DAPT
- dual antiplatelet therapy
- DC
- DOAC plus clopidogrel
- DOAC
- direct oral anticoagulants
- ICH
- intracerebral hemorrhage
- PCI
- percutaneous coronary intervention
- TT
- triple therapy
Carotid stenosis (CS) represents approximately 12%–19.3% of acute ischemic strokes.1 During the past decade, carotid artery stent placement (CAS) has emerged as an alternative treatment option for CS when carotid endarterectomy is not feasible or as a first-line therapy.2 After CAS, patients should follow a dual antiplatelet therapy (DAPT) regimen with aspirin and clopidogrel from 1–3 months to minimize the risks of acute stent thrombosis secondary to platelet activation triggered by intimal injury and/or insertion of a foreign body (stent).3 However, when atrial fibrillation (AF) coexists, the best approach is yet to be elucidated. According to a recent metanalysis, 12% of patients with AF have a moderate-severe CS, which doubles the risk of stroke in patients with AF after adjustment for classic clinical risk factors and antithrombotic therapy.4,5
In this setting, DAPT plus oral anticoagulation is expected to be the most effective approach, but with an increment of bleeding rates. On the other hand, oral anticoagulation or DAPT alone may not be effective enough to prevent stent thrombosis or embolic events, respectively. Nevertheless, no clinical trials have addressed this question before. By contrast, 3 randomized clinical trials including patients with AF and a recent acute coronary syndrome or percutaneous coronary intervention (PCI) treated with a P2Y12 inhibitor and direct oral anticoagulants (DOACs) without aspirin resulted in less bleeding without an increment in the incidence of ischemic events compared with regimens that included a vitamin K antagonist, aspirin, or both.6⇓-8
Our aim was to compare 3 different antithrombotic regimens: DOAC plus a P2Y12 inhibitor, anticoagulation plus DAPT, or DAPT alone in patients undergoing CAS with concomitant AF in a daily clinical practice.
MATERIALS AND METHODS
Study Strategy
The present study is a retrospective observational single-center study including patients undergoing CAS in a comprehensive stroke center from 2010 to March 2021. We included patients who met all the following criteria: previous, persistent, permanent, or paroxysmal nonvalvular AF; TIA or minor/mild stroke secondary to atherosclerotic CS who underwent CAS as secondary prevention or asymptomatic CS with revascularization criteria; and planned use of a P2Y12 inhibitor for at least 1 month. To stratify the risk of stroke among patients with AF, we assessed the CHA2DS2-VASc scores. The bleeding risk was measured by the HAS-BLED scores.
Data Collection
Baseline variables and ultrasound findings were prospectively recorded. Outcomes were retrospectively collected. The reporting of this study conforms to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE; https://www.strobe-statement.org/) statement (for the STROBE checklist of this study). The study was approved by the local ethics committee (University Hospital Virgen del Rocío Ethic Committee/1431-N-21) and followed the Declaration of Helsinki Ethical principles of medical research involving human subjects.
Regimen Strategy
We established 3 groups according to antithrombotic regimen: 1) DOAC plus clopidogrel (DC group), 2) DAPT with clopidogrel and aspirin plus anticoagulation (triple therapy [TT] group), and 3) DAPT with clopidogrel and aspirin alone (DAPT group). The direct oral anticoagulant doses administered to the DC group were rivaroxaban, 15 mg once daily, apixaban, 5 mg twice daily (2.5 mg daily if dose-reduction criteria were required), and dabigatran, 110 mg twice daily. The antithrombotic regimen was established by stroke physician criteria 24 hours after CAS. Clopidogrel was stopped at 1 month after CAS in patients included in the TT and DAPT groups and was switched to aspirin in patients in the DC group.
CAS
Patients were treated with double antithrombotic therapy for at least 5 days (aspirin, 100 mg/day, and clopidogrel, 75 mg/day) before CAS or were treated with a loading dose of clopidogrel (300 mg) and aspirin (300 mg). Anticoagulation was stopped 24 hours before CAS and restored within 24 hours after CAS, except in those treated with DAPT alone. Predilation of the stenosis with a 2- to 4-mm balloon catheter was performed previous to a self-expanding stent being deployed. Postdilation with a 5- to 6-mm balloon was performed in cases with residual stenosis. Heparin was administered systematically during the procedure to maintain an activated clotting time between 250 and 300 seconds.
Follow-up Assessments
Neurologic and systemic evaluation was performed the day before the procedure, immediately after CAS, 24 hours after intervention, and at 1-month follow-up. On days 1 and 30 after CAS, a carotid ultrasound was performed to rule out stent thrombosis or restenosis.
Outcome Measures
The safety end point was major or clinically relevant nonmajor bleeding as defined by the International Society on Thrombosis and Haemostasis. Major bleeding was defined by the International Society on Thrombosis and Haemostasis as bleeding that resulted in death, occurred in a critical organ (intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, intramuscular with compartment syndrome, or pericardial), or was associated with either a decrease in the hemoglobin level of at least 2 g/dL or a transfusion of at least 2 U of packed red cells. Clinically relevant nonmajor bleeding was defined as bleeding that resulted in hospitalization, medical or surgical intervention for bleeding, an unscheduled clinic visit, or a change in physician-directed antithrombotic therapy.9
The efficacy end point was stroke recurrence at 30 days from CAS or any ischemic event (myocardial infarction, stent thrombosis, stroke, and systemic embolism).
Statistical Analysis
Values for continuous variables with a normal distribution are presented as mean (SD), and 2-sample t tests were performed for comparisons. The Mann-Whitney test and medians and interquartile ranges were used for non-normal distributions. Categoric variables were analyzed with the χ2 test or the Fisher exact test, as appropriate. We used SPSS 25.0 (IBM), with P < .05 considered statistically significant.
RESULTS
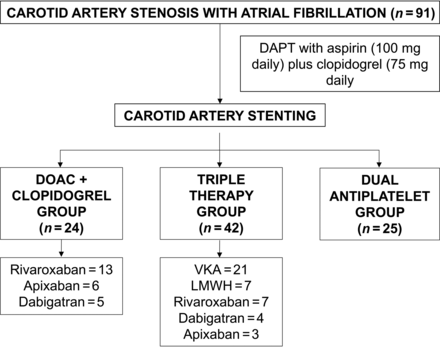
We analyzed 959 patients from 2010 to March 2021, of whom 91 met all the inclusion criteria (DC group = 24 patients, TT group = 42 patients, and DAPT group = 25 patients). Sixty-nine patients (75.82%) had symptomatic CS (DC group = 21, TT group = 31, and DAPT group = 17). The baseline characteristics are summarized in Table 1. The mean age was 72.27 (SD, 8.1) years, and 17.6% of the patients were women. The mean CHAsDS2-VASc score was 5.3 (SD, 1.3), and the mean HAS-BLED score was 3.9 (SD, 0.9). Among patients in the DC group, rivaroxaban was administered in 13 patients; apixaban, in 6 patients; and dabigatran, in 5. In TT group, 21 patients received vitamin K antagonists; 7 patients, low-molecular-weight heparin; and 14, DOAC (7 rivaroxaban, 4 dabigatran, and 3 apixaban). Clopidogrel was the P2Y12 inhibitor in the 3 groups (Fig 1).
Baseline characteristics
Antithrombotic strategy management. LMWH indicates low-molecular-weight heparin; VKA, vitamin K antagonist.
Safety Outcomes
At 1 month, 10 of 42 patients (23.8%) receiving triple therapy had a major or clinically relevant nonmajor bleeding event, compared with 1 of 24 (4%) receiving DAPT (P = .032) and no bleeding event in the DC group (P = .007) (Table 2). A major bleeding did not occur either in the DC or DAPT group, compared with 4 major bleeding events in the TT group (Online Supplemental Data). An intracranial hemorrhage was reported in the TT group.
Safety and efficacy outcomes
Efficacy Outcomes
Neither systemic embolisms, myocardial infarction, nor stroke were reported in the TT group at 1 month after CAS. One patient in the DC group was hospitalized with minor stroke symptoms due to carotid stent thrombosis. In a patient included in DAPT group in whom a CAS in the right carotid artery was performed, a cardioembolic stroke involving the contralateral MCA was reported (Table 2).
DISCUSSION
To the best of our knowledge, our study evaluates, for the first time, the safety and efficacy of 3 different antithrombotic regimens in patients undergoing CAS with concomitant AF, including a combination of DOAC plus clopidogrel. Our study showed that the regimen of DOAC plus clopidogrel during the first month resulted in a lower risk of a major or clinically relevant nonmajor bleeding event than the usual regimen with triple therapy, with no increment of thromboembolic events.
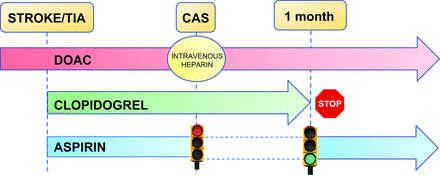
Regimens including DOAC therapy appear to offer the potential for less bleeding over the vitamin K antagonists in patients who underwent PCI.10 The Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention (PIONEER AF-PCI) trial showed that a regimen involving low doses of rivaroxaban (15 mg once daily or 2.5 mg twice daily) plus a P2Y12 inhibitor had a lower incidence of bleeding rates compared with a regimen of vitamin K antagonist and DAPT.7 The Randomized Evaluation of Dual Anti- thrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (RE-DUAL PCI) trial demonstrated that a regimen of dabigatran plus a P2Y12 inhibitor conferred lower rates of bleeding than triple therapy.8 Similar results were seen in the AUGUSTUS trial, in which the use of apixaban plus a P2Y12 inhibitor had a better safety profile than regimens that included a vitamin K antagonist, aspirin, or both.6 The decrement of bleeding risk was accompanied by similar thrombotic events as well. Because patients having undergone CAS usually have a higher risk of bleeding than patients having undergone PCI, the combination of DOAC plus clopidogrel might be also effective in our patients (Fig 2).
Management recommendations in patients undergoing CAS with concomitant AF. Once CS (if suitable for CAS) is diagnosed, we recommend dual antiplatelet therapy (aspirin and clopidogrel) plus anticoagulation with a DOAC at least 5 days before CAS. Anticoagulation should be stopped 24 hours before CAS and re-initiated 24 hours after. According to antiplatelet therapy after CAS, we recommend a regimen including DOAC plus clopidogrel during the first month after CAS and then switching clopidogrel to aspirin.
For the primary safety outcome of major or clinically relevant nonmajor bleeding at 30 days, a significant difference risk of 23.8% between a regimen of DOAC plus clopidogrel and a triple therapy was detected. Major bleeding was reported neither in the DC group nor the DAPT group. Patients included in our study had a specifically high risk of intracerebral hemorrhage (ICH) due to the following reasons: 1) recent previous stroke symptoms, 2) risk of developing hyperperfusion syndrome, and 3) a high HAS-BLED score. Nevertheless, ICH occurred in neither the DC group nor the DAPT group. Our findings support this regimen possibly providing a good safety profile in patients with AF in whom CAS is performed. There were no statistically significant differences in efficacy end points. However, a stent thrombosis that occurred in the DC group might have been related to insufficient antiplatelet therapy or clopidogrel resistance. Also, a stroke was observed in the DAPT group, probably related to the absence of anticoagulation therapy. Our results suggest a balance of the risk of bleeding and the prevention of stent thrombosis or embolic events.
To our knowledge, there is only 1 report addressing this question to compare with our results.11 The authors included 31 patients, of whom 14 received a DOAC plus a single antiplatelet therapy (aspirin or clopidogrel) with no bleeding event described. By contrast, in only 1 of 17 (5.9%) patients receiving vitamin K antagonists plus DAPT was a bleeding event reported. The low bleeding event rate might be related to either the INR target (1.5–2) compared with our higher INR target (2–3) or to the smaller sample size.
Our study has several limitations. This was a retrospective, nonrandomized study in which the choice of each antithrombotic regimen was uncontrolled. However, the treatment eligibility criteria were not related to bleeding or thrombotic risk because CHA2DS2-VASC and HAS-BLED scores were similar within groups, but they were due to publication of this new regimen in patients with AF undergoing PCI because 87.5% of patients in the DC group were included from 2017. In addition, we did not perform an evaluation of antiplatelet effects in our patients. Although the small sample size impedes obtaining robust evidence in terms of efficacy compared with triple therapy, it may allow setting up a hypothesis of a novel management in patients undergoing CAS with AF.
CONCLUSIONS
In patients who are eligible for CAS with concomitant AF, a regimen of triple therapy confers a high bleeding risk. Another regimen such as DOAC plus a P2Y12 inhibitor, similar to that in patients undergoing PCI with concomitant AF, might confer a good safety profile with an optimal efficacy. A randomized clinical trial should be conducted to confirm the efficacy and safety of this therapy.
Footnotes
This work was supported by the Instituto de Salud Carlos III (PI15/01197, PI18/01414, RD16/0019/0015 and CM21/00096), and cofunded by the European Regional Development Fund/European Social Fund, “A way to make Europe"/"Investing in your future”.
B. Pardo-Galiana and M. Medina-Rodriguez contributed equally to this work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received December 14, 2021.
- Accepted after revision February 9, 2022.
- © 2022 by American Journal of Neuroradiology