Abstract
BACKGROUND AND PURPOSE: Intracranial atherosclerotic plaque features are potential factors associated with recurrent stroke, but previous studies only focused on a single lesion, and few studies investigated them with perfusion impairment. This study aimed to investigate the association among whole-brain plaque features, perfusion deficit, and stroke recurrence.
MATERIALS AND METHODS: Patients with ischemic stroke due to intracranial atherosclerosis were retrospectively collected and categorized into first-time and recurrent-stroke groups. Patients underwent high-resolution vessel wall imaging and DSC-PWI. Intracranial plaque number, culprit plaque features (such as plaque volume/burden, degree of stenosis, enhancement ratio), and perfusion deficit variables were recorded. Logistic regression analyses were performed to determine the independent factors associated with recurrent stroke.
RESULTS: One hundred seventy-five patients (mean age, 59 [SD, 12] years; 115 men) were included. Compared with the first-time stroke group (n = 100), the recurrent-stroke group (n = 75) had a larger culprit volume (P = .006) and showed more intracranial plaques (P < .001) and more enhanced plaques (P = .003). After we adjusted for other factors, culprit plaque volume (OR, 1.16 per 10-mm3 increase; 95% CI, 1.03–1.30; P = .015) and total plaque number (OR, 1.31; 95% CI, 1.13–1.52; P < .001) were independently associated with recurrent stroke. Combining these factors increased the area under the curve to 0.71.
CONCLUSIONS: Large culprit plaque and more intracranial plaques were independently associated with recurrent stroke. Performing whole-brain vessel wall imaging may help identify patients with a higher risk of recurrent stroke.
ABBREVIATIONS:
- HR-VWI
- high-resolution vessel wall imaging
- IQR
- interquartile range
Intracranial atherosclerosis is the primary etiology of stroke in Asia,1 and the risk of recurrent stroke in these patients is high even under aggressive medication.2 Identification of the risk factors associated with recurrent stroke is essential for secondary stroke prevention.
The degree of stenosis is a well-recognized predictor of recurrent stroke, and patients with >50% stenosis are recommended for more aggressive treatment.3,4 However, vulnerable atherosclerotic plaques with <50% stenosis can also lead to stroke events.5,6 The development of high-resolution vessel wall imaging (HR-VWI) has enabled the visualization of vulnerable features (intraplaque hemorrhage, outward remodeling, neurovascularture) in vivo and has provided important insight into plaque vulnerability rather than the degree of stenosis alone.7,8 The use of whole-brain HR-VWI also allows the evaluation of multiple lesions simultaneously.9
Previous HR-VWI studies of intracranial plaques focused on comparing patients with stroke and asymptomatic patients.10,11 However, investigations of intracranial plaque features associated with recurrent stroke are still rare. A few studies found that plaque enhancement12 and plaque burden13 were associated with stroke recurrence. However, these studies focused on MCA stenosis rather than whole-brain evaluation. Recent studies found that the total plaque number was a novel imaging marker of intracranial atherosclerosis;14,15 evaluating the entire cerebral arterial tree may provide additional information. However, the relationship with stroke recurrence was not studied in these research. Perfusion impairment is also related to recurrent stroke16 but has been rarely studied together with whole-brain HR-VWI features.16
Our study aimed to evaluate intracranial total plaque number by whole-brain HR-VWI; explore the difference in intracranial culprit plaque features, total plaque number, and perfusion deficit between first-time and recurrent stroke groups using HR-VWI and DSC-PWI; and find the association between these factors with stroke recurrence.
MATERIALS AND METHODS
Study Patients
This retrospective single-center observational study was approved by the Ethics Committee of Tianjin First Central Hospital, and the requirement of patient informed consent was waived. From September 2016 to September 2020, the patients who underwent HR-VWI in our hospital and met the following inclusion criteria were selected, and their clinical and MR imaging data were reviewed. Inclusion criteria were the following: 1) older than 18 years of age; 2) ischemic stroke in the unilateral MCA or posterior circulation territory based on positive findings on DWI; 3) DSC-PWI included in the MR imaging protocol; 4) all MR images acquired at the same scan session within 30 days of symptom onset; 5) intracranial atherosclerosis considered as the etiology of stroke events based on the following: confirmation of intracranial large arterial atherosclerotic plaque upstream of the stroke territory, identification of atherosclerotic plaque on the parent artery for patients with an isolated infarct in the penetrating artery territory, no imaging evidence of aortic arch atherosclerosis, no clinical or imaging evidence of cardioembolism, and exclusion of other etiologies by relevant clinical history and examination; and 6) complete clinical data and satisfactory imaging quality. Exclusion criteria included the following: 1) patients with recurrent events in the territories different from the previous ischemic territories; 2) acute lacunar infarction on DWI; 3) acute ischemic stroke involving other arterial territories (anterior cerebral artery, bilateral circulation, both anterior and posterior circulation); 4) nonatherosclerosis vasculopathy; 5) evidence of cardioembolism (such as recent myocardial infarction within 1 month, atrial fibrillation, mitral stenosis, prosthetic valve thrombosis, and endocarditis); 6) ≥50% stenosis or the presence of vulnerable carotid plaques (the presence of intraplaque hemorrhage, large lipid-rich necrotic core, or irregular surface) of the ipsilateral extracranial carotid artery on HR-VWI; 7) complex aortic arch plaque (plaque of ≥4 mm or with irregular ulceration) confirmed by CTA; and 8) poor imaging quality and incomplete clinical data. Clinical information, including age, sex, vascular risk factors (the diagnostic criteria are shown in the Online Supplemental Data), preadmission statin use, symptom onset to HR-VWI interval, NIHSS score on admission, and several blood biochemical indexes, was recorded for each patient.
Patient Groupings
Patients were divided into the recurrent-stroke group and the first-time stroke group, and there were no overlapping patients between these 2 groups. The patients with recurrent-stroke events met the following criteria: acute neurologic deficit on admission that fitted the definition of acute ischemic stroke, high-intensity signal on DWI, previous ischemic stroke history confirmed by hospital records, and corresponding encephalomalacia in the same territory on FLAIR.17,18 The symptoms and signs of initial strokes had resolved before the symptom onset of recurrent-stroke events.17 The patients in the first-time stroke group had the same criteria as the recurrent-stroke group, except for the previous stroke history and corresponding encephalomalacia on FLAIR.
Imaging Protocol
All MR images were obtained on a 3T whole-body system (Magnetom Prisma; Siemens) with a 64-channel head-and-neck coil. The imaging protocol included DWI, FLAIR, strategically acquired gradient-echo imaging,19 precontrast T1-weighted HR-VWI, DSC-PWI, and postcontrast HR-VWI (parameters are detailed in the Online Supplemental Data). DWI helped the localization of the acute cerebral ischemic lesion. FLAIR was used to confirm the remote cerebral infarction. DSC-PWI was used for the evaluation of perfusion deficits. HR-VWI was performed using the inversion recovery prepared sampling perfection with application-optimized contrast by using different flip angle evolutions (SPACE)20 sequence in a sagittal plane optimized for intracranial plaque evaluation and flow signal suppression. The SPACE sequence was nonselective excitation and covered the whole brain. DSC-PWI was performed with contrast agent injection (0.2 mL/kg; 0.9% sodium chloride flush, 15 mL; flush rate, 2 mL/s) using gadobenate dimeglumine. Postcontrast HR-VWI was performed after DSC-PWI.
Imaging Analysis
Quantitative analysis of plaque features (except plaque volume) was performed using the PACS. The plaque volume was measured semiautomatically using commercial volumetric analysis software, ITK-SNAP 3.8.0 (www.itksnap.org).21 The perfusion evaluation was performed using commercial software, Fast-Processing of Ischemic Stroke software (F-STROKE; Version 1.0.18; Neuroblem). MR imaging analyses were performed by 2 neuroradiologists (G.W. and C.Z. with 8 and 6 years of experience, respectively). The reviewers had access to the source images and reconstructed images but were blinded to the clinical information.
Plaque Identification.
Due to the small size of the intracranial arteries, only the large arteries were analyzed, including intracranial ICAs (cavernous and supraclinoid segments), A1 and A2 segments of the anterior cerebral arteries, M1 and M2 segments of MCAs, P1 and P2 segments of posterior cerebral arteries, basilar arteries, and intracranial segments of the vertebral arteries. An atherosclerotic plaque was identified as focal vessel wall thickening with reference to adjacent proximal, distal, or contralateral vessel segments,22 regardless of whether it caused luminal stenosis. Multiple plaques could be on the same arterial segment, and the determination of multiple plaques was as follows (Online Supplemental Data): Two plaques were considered as 2 separate plaques if they were discontinuous and had a normal arterial wall between them. The plaques that involved several segments were counted as separate plaques for each segment.
Plaque Classification.
A plaque located upstream of the infarct lesion was defined as a culprit plaque when it was the only plaque within the vascular territory of the stroke or the most stenotic plaque when multiple plaques existed within the same vascular territory of the stroke.22 A plaque was considered a probably-culprit plaque if it was within the vascular territory but not the most stenotic plaque.22 A plaque was defined as nonculprit plaque if it was not within the vascular territory of stroke.22 During the identification of plaque, any disagreements between the 2 reviewers were resolved by discussion and consensus under the guidance of a senior neuroradiologist (Y.G. with 17 years of experience in neuroradiology).
Plaque Feature Measurement.
By means of the multiplanar reformation function of the PACS, the lumen and vessel boundaries were manually segmented (Online Supplemental Data) at the most stenotic location of the plaque on the reformatted cross-sectional postcontrast HR-VWI. For the evaluation of intraplaque hemorrhage and plaque-enhancement grading, the plaque was observed section by section on the reformatted images of precontrast and postcontrast HR-VWI. The following parameters of culprit plaque and probably-culprit plaque were derived from the measurements:
Plaque area = vessel area – lumen area.
Plaque burden = (1–lumen area/vessel area) × 100%.23
Arterial remodeling ratio = vessel area/reference vessel area. The arterial remodeling pattern at the lesion site was categorized as positive remodeling if the arterial remodeling ratio was >1.05.23
Eccentric index = (1–minimum wall thickness/maximum wall thickness) × 100%. The plaque was classified as eccentric if the eccentric index was ≥0.5, and concentric if <0.5.24
A plaque with intraplaque hemorrhage was defined if the signal intensity of its brightest part on the precontrast HR-VWI was higher than 150% of the signal intensity of the reference vessel wall.25
With precontrast and postcontrast HR-VWI, the enhancement ratio13 and enhancement grading22,26 of plaque were analyzed using previously established methods,13,22,26 detailed in the Online Supplemental Data. A plaque was considered enhanced if the enhancement degree was grades 1 and 2.
The degree of stenosis = (1–lumen diameter/reference lumen diameter at the normal proximal site) × 100%.27
The plaque volume was measured semiautomatically using ITK-SNAP 3.8.0 software. On the cross-sectional slices of postcontrast HR-VWI, the plaque boundaries were delineated section by section, and the volume of each plaque was recorded (Online Supplemental Data).
All the above plaque features were evaluated for the culprit and probably-culprit plaques, but only the enhancement grading was analyzed for nonculprit plaques. In addition, intracranial total plaque number and enhanced plaque number were recorded for each patient.
Perfusion Deficit Evaluation.
By means of Fast-Processing of Ischemic Stroke software (F-STROKE; Version 1.0.18), the time-to-maximum volumes with different thresholds were automatically calculated. Three different variables were recorded for each patient as follows:
The hypoperfusion volume was defined as the hypoperfusion volume of time-to-maximum with a threshold of >6 seconds.28
Hypoperfusion intensity ratio = (the hypoperfusion volume of time-to-maximum with a threshold of >10 seconds)/(the hypoperfusion volume of time-to-maximum with a threshold of >6 seconds).29
Hypoperfusion existed if the hypoperfusion volume was >0 mL.
Statistical Analysis
The clinical and imaging features of the 2 groups were analyzed. The frequency and distribution of plaques per arterial segment were also explored. Continuous variables were provided using mean (SD) or median (interquartile range [IQR]). Categoric variables were presented as frequencies. All group differences of culprit plaque features and perfusion deficits were compared using the Student t test, Mann-Whitney U test, or χ2 test as appropriate. Because the same patient might have multiple probably-culprit plaques, the generalized estimating equations with a robust covariance matrix estimator were used for the differences in probably-culprit plaque features between the first-time and recurrent-stroke groups. Clinical and imaging variables with a P value < .1 from the univariable analysis were included in the following analysis: Multivariable logistic regression analysis (forward likelihood ratio method) was used for determining the independent factors associated with patients with recurrent stroke. Reproducibility assessment was evaluated using intraclass correlation coefficients (continuous variables) (Online Supplemental Data). Two-sided P < .05 indicated statistical significance in all analyses. The receiver operating characteristic curves were analyzed for parameters with independent significance. The Delong test was performed for comparison of the area under the curve values. SPSS 24.0 (IBM) and MedCalc 19.0.4 (MedCalc Software) were used for all analyses.
RESULTS
Patient Characteristics
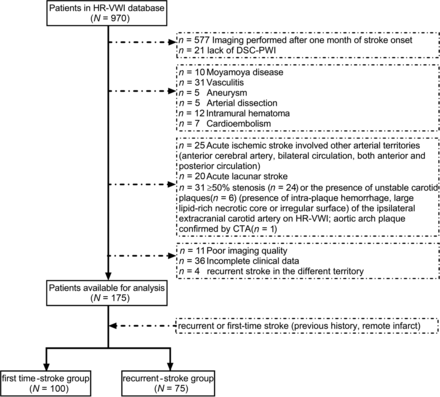
From September 2016 to September 2020, one hundred seventy-five patients (mean age, 59 [SD, 12] years; 115 men) were included in the final analysis (Fig 1). As Table 1 shows, the median interval between symptom onset and HR-VWI was 10 (IQR, 6–15) days. Among these patients, 75 patients had recurrent stroke and 100 patients had first-time stroke. The median stroke recurrence time was 15.0 (IQR, 11.0–21.0) months in the recurrent-stroke group. Patients with recurrent stroke had a higher proportion of preadmission statin use (22.7% versus 7.0%, P = .003) than those with first-time stroke. Age, sex, conventional risk factors, the interval between symptom onset and HR-VWI, NIHSS score, blood biochemical indexes, and stroke event frequencies in different vascular territories were not statistically different between these 2 groups.
Flow chart of the study population and patient grouping.
Clinical characteristics of the study population
Reproducibility of Imaging Measurements
There was good interreviewer and intrareviewer agreement with all intraclass correlation coefficients being ≥0.89 (Online Supplemental Data).
Plaque Distribution and Locations
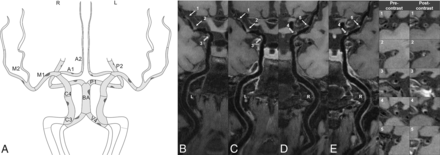
A total of 786 atherosclerotic plaques were identified in 175 patients. A mean number of 4.5 (SD, 2.3) plaques was identified per patient, with 2.8 (SD, 1.4) plaques in the anterior circulation and 2.6 (SD, 1.4) plaques in the posterior circulation (detailed in Fig 2 and the Online Supplemental Data). In the recurrent-stroke group, 53 patients had anterior circulation stroke events, with 44 (83.0%) culprit plaques in the MCAs; 10 (45.4%) culprit plaques were detected in the basilar arteries in 22 cases of posterior circulation stroke. In the first-time stroke group, 71 patients had anterior circulation stroke events, with 66 (93.0%) culprit plaques in the MCAs; 13 (44.8%) culprit plaques were detected in the basilar arteries in 29 cases of posterior circulation stroke. The distribution of culprit plaques showed no statistical difference between the 2 groups (P = .36, Online Supplemental Data). In addition, 54 and 43 probably-culprit plaques were identified in the recurrent- and first-time stroke groups, respectively.
Intracranial plaque distribution and frequency. The schematic figure (A) shows analyzed artery segments with the frequency of intracranial plaques. The segments are shown in light gray: intracranial ICAs (cavernous/C3 and supraclinoid/C4 segments), A1 and A2 segments, M1 and M2 segments, P1 and P2 segments, basilar artery (BA), and intracranial vertebral arteries (V4). The frequency of plaques in the cohort (n = 175) is illustrated through plaques. A large dark-gray plaque indicates a higher frequency of plaques within the specific segment. The detailed results are listed in the Online Supplemental Data. The HR-VWI shows a patient with 5 intracranial plaques. Precontrast images indicate 2 left M1 plaques (B, arrows), 1 left ICA plaque (B, hollow arrow), 1 right M1 plaque (D, arrow), and 1 right ICA plaque (D, hollow arrow). Postcontrast HR-VWI (C and E) shows that the plaques are enhanced in different degrees. The last 2 columns show the cross-sectional views of each plaque. L indicates left; R, right.
Imaging Features between Patients with First-Time and Recurrent Stroke
All the imaging features of 175 patients and comparisons between the 2 groups are shown in Table 2. The recurrent-stroke group had culprit plaques with larger volumes (P = .006) compared with the first-time stroke group. No significant difference in other culprit plaque features was detected between the 2 groups (plaque area, P = .40; plaque burden, P = .26; arterial remodeling ratio, P =.83; positive remodeling, P = .92; eccentric index, P = .54; eccentricity, P = .56; intraplaque hemorrhage, P = .68; degree of stenosis, P = .23; enhancement ratio, P = .89). Compared with the first-time stroke group, the recurrent-stroke group showed significantly more intracranial plaques per patient (median, 5 lesions; IQR, 4–7 lesions versus median, 4 lesions, IQR, 2–5 lesions; P < .001) and more enhanced intracranial plaques per patient (median, 3 lesions; IQR, 2–4 lesions versus median, 2 lesions; IQR, 1–3 lesions; P = .003). No significant difference in perfusion deficit variables and HR-VWI features of probably-culprit plaques was detected between the 2 groups (Table 2 and Online Supplemental Data, all P > .05).
Imaging features of the study population
Association between Imaging Features with Patients with Recurrent Stroke
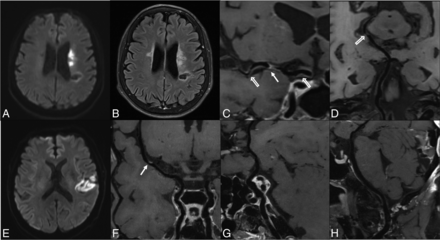
As Table 3 shows, culprit plaque volume, total plaque number, and enhanced plaque number were included in the multivariable analysis. After multicollinearity diagnosis (Online Supplemental Data), culprit plaque volume (OR, 1.16 per 10-mm3 increase; 95% CI, 1.03–1.30; P = .015) and total plaque number (OR, 1.31; 95% CI, 1.13–1.52; P < .001) were the imaging features independently associated with patients with recurrent strokes. The area under the curve value was 0.62 (95% CI, 0.54–0.70) for culprit plaque volume, 0.66 (95% CI, 0.59–0.74) for total plaque number, and 0.71 (95% CI, 0.61–0.79) for combining 2 factors with a sensitivity of 84.0% and specificity of 53.0%. The combined model increased the area under the curve to 0.71, higher than any variable alone (all, P < .05). The representative cases are shown in Fig 3.
Representative patients of recurrent- and first-time stroke groups. Upper row, A 64-year-old female patient with right-limb weakness and inarticulateness for 5 days. MR imaging was performed 9 days after symptom onset. DWI (A) shows a left basal ganglia infarction, FLAIR (B) shows a remote infarction on the left centrum semiovale, and HR-VWI (C and D) indicates M1 culprit plaque (arrow) (39.4 mm3), and other plaques detected in the left MCA, internal carotid artery, and left posterior cerebral artery (hollow arrows). The total plaque number is 5. Lower row, A 63-year-old male patient who presented with sudden abasia and inarticulateness for 2 hours. MR imaging was performed 5 days after the symptom onset. DWI (E) shows left insular and temporal lobe infarction, HR-VWI (F) shows a culprit plaque in the left M1 segment (arrow) (18.6 mm3), and no other plaques (G and H) are detected in other intracranial arteries. The total plaque number is 1.
Univariable and multivariable analysis to identify parameters associated with patients with recurrent stroke compared with patients with the first-time stroke
DISCUSSION
There were 3 important findings in the current study. First, HR-VWI was used to investigate the whole-brain plaque in patients with first-time and recurrent stroke; and it had additional values compared with investigating a single lesion. Second, patients with recurrent stroke presented with larger culprit plaque, more intracranial plaques, and more enhanced intracranial plaques compared with patients with first-time stroke. Third, culprit plaque volume and intracranial total plaque number were 2 independent features associated with patients with stroke recurrence by HR-VWI measurement. Combining culprit plaque volume and total plaque number might improve the differentiation between patients with recurrent and first-time stroke. Our study highlights the importance of performing whole-brain vessel wall imaging and evaluating the atherosclerotic plaques in the entire large cerebral arterial tree.
Atherosclerosis is a systemic disease that involves multiple vascular beds. The average number of intracranial plaques in our cohort was 4.5 per patient. Previous studies using 7T MR imaging found 4.9 or 4.5 plaques per patient in the symptomatic patients of the same age.15,30 Although 7T scanners performed better in detecting lesions, we believe that the discrepancy was probably due to the susceptibility of Chinese patients to intracranial artery stenosis, with earlier onset age and higher prevalence.31
In our study, patients with recurrent-stroke events had larger culprit plaques (measured by plaque volume) than those with first-time stroke events. No significant difference in other culprit plaque features such as the degree of stenosis,4 plaque enhancement,12,32 and plaque burden13 was detected between the 2 groups, which was also reported by a recent similar study.16 Plaque burden13 and its progression with time33 were reported to be associated with stroke recurrence. Although plaque burden might cover both luminal stenosis and outward remodeling changes, the information based on measurement from 1 section might be very limited. Instead, plaque volume would be more representative of the actual plaque burden; the result would also indicate the value of HR-VWI in evaluating arterial wall changes. A longitudinal MR imaging study showed that the progression of carotid plaque volume was independently associated with recurrent cerebral ischemic events.34 In contrast, another follow-up HR-VWI study found that the progression of intracranial culprit plaque burden rather than the progression of plaque volume was associated with recurrence.33 Our results differed from the latter, possibly because they included patients with both stroke and transient ischemic attack, but we only focused on patients with stroke; their analysis was based on 2D imaging data, making the measurement not accurate enough, while our study adopted 3D whole-brain scanning. Longitudinal studies using 3D HR-VWI are still needed to explore the association between plaque volume and stroke recurrence. The Warfarin versus Aspirin for Symptomatic Intracranial Disease trial showed that >70% stenosis predicted recurrent stroke.4 Our study did not show the degree of stenosis independently associated with the recurrent-stroke group, possibly because most of our study population had moderate stenosis (median, 44.7%; IQR, 31.3%–63.6%). These plaques without severe stenosis could also lead to stroke, a finding that agreed with those in a recent meta-analysis.5
Several recent studies also demonstrated that culprit plaque enhancement or enhancement ratio was related to recurrent stroke.12,13,32 Our study quantitatively analyzed the culprit enhancement ratio but did not get a similar result. Instead of culprit plaque enhancement, our study indicated that the patients with recurrent-stroke events had more enhanced plaques, which might indicate more vulnerable plaques. The underlying reasons might be poorly controlled systemic risk factors, hemodynamic abnormality, inflammatory reaction, and corresponding physiologic and biochemical reactions, which would aggravate and facilitate the progression of atherosclerosis.35,36 Also, the median stroke-recurrence time was 15.0 (IQR, 11.0–21.0) months in the recurrent-stroke group, and that might be long enough to ignore the effect of the previous strokes on the plaque enhancement.37
Although no significant difference was detected in these features (except plaque volume) in our study, the evaluation of vulnerable plaque features was still necessary. For example, the plaque with large volumes might easily block penetrating arteries leading to infarct. Also, the vulnerable plaques were inclined to rupture, resulting in artery-to-artery embolism, and the stenotic plaques could cause hypoperfusion downstream. The retrospective nature might be a reason for the insignificance of our study; future larger-scale prospective studies are needed to identify the plaque vulnerability and its association with stroke recurrence.
The intracranial total plaque number was independently associated with the recurrent -stroke group in our study. Investigators in the Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE) study3 found higher stroke recurrence at 90 days among patients with multiple intracranial artery stenoses (>50%) identified on MRA than those without. The Chinese IntraCranial AtheroSclerosis (CICAS) study38 found that the patients in the group with medium intracranial atherosclerosis burden showed more stroke recurrence at 12 months (adjusted hazard ratio, 1.96; P = .027) with more patients with mild stenosis in this group. These previous studies using TOF-MRA indicated the necessity of analyzing the entire large cerebral artery tree and of the plaques with mild stenosis needing to be fully accessed. TOF-MRA as a conventional luminal imaging method could have flow artifacts, overestimate the luminal stenosis, or miss mild stenotic plaques; even DSA would miss up to 26.1% of nonstenotic plaques.8 Compared with angiographic imaging, HR-VWI performed better because it could quantify luminal stenosis accurately and reproducibly,8 detect more nonstenotic plaques,8 and evaluate several vulnerable features. Moreover, our study adopting the whole-brain HR-VWI scan was capable of the simultaneous evaluation of large cerebral arteries. Our results, more intracranial plaques in patients with recurrent stroke, may indicate a heavy atherosclerosis burden and poor systemic disease control in our patients.14,15
Our study also evaluated several perfusion deficit variables, but no difference was detected between groups. The perfusion deficit in patients with intracranial atherosclerosis could lead to stroke events and was related to recurrent stroke. The cerebral hypoperfusion downstream due to stenotic plaques or artery-to-artery embolism caused by ruptured vulnerable plaques might result in cerebral infarct.39 A recent study on a similar topic using arterial spin-labeling found that the hypoperfusion volume ratio, which correlated with collateral grades, was associated with stroke recurrence.16 Their findings were different from ours, possibly due to the cross-sectional retrospective nature of our study and the arterial spin-labeling perfusion imaging with multiple postlabelling delays they used possibly providing information about both hypoperfusion and collateral flow.
There were several limitations to this study. First, although culprit plaque volume and plaque number were independent imaging factors associated with patients with recurrent stroke in our cohort, these findings may be coincidental. Ischemic stroke due to intracranial atherosclerosis is a multifactorial disease; the imaging markers we explored may result from multiple clinical factors. The future evaluation of stroke risk should be more comprehensive. Second, our study was retrospective. The measurement of plaque features probably happened after the plaque rupture, so the associations between plaque features and recurrence needed further evaluation in the prospective study. Third, our study was cross-sectional. The factors associated with patients with recurrent stroke might not present in the first-time stroke as baseline features. Longitudinal studies are needed to confirm our findings. Last, due to the long scan time of the MR imaging protocols, only patients without severe symptoms were enrolled, probably leading to more patients with low NIHSS scores in our cohort. In addition, this study did not include extracranial carotid plaques; future studies applying large-coverage head-and-neck HR-VWI on concomitant intracranial and extracranial atherosclerosis would provide additional insights.40
CONCLUSIONS
Large culprit plaque and more intracranial atherosclerotic plaques were independently associated with recurrent stroke. Performing whole-brain vessel wall imaging may help better identify patients with a higher risk of recurrent stroke.
Footnotes
Shuang Xia and Chengcheng Zhu contributed equally to this study as co-senior authors.
This work was supported by the National Natural Science Foundation of China (grant No. 81871342 to S. Xia and grant No. 81901728 to C. Chai), the Natural Science Foundation of Tianjin (grant No. 20JCQNJC01250 to C. Cao), and the National Key R&D Programme of China (grant No. 2019YFC0120903 to S. Xia).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received July 14, 2021.
- Accepted after revision November 2, 2021.
- © 2022 by American Journal of Neuroradiology