Abstract
BACKGROUND AND PURPOSE: White matter lesions of presumed ischemic origin are associated with progressive cognitive impairment and impaired BBB function. Studying the longitudinal effects of white matter lesion biomarkers that measure changes in perfusion and BBB patency within white matter lesions is required for long-term studies of lesion progression. We studied perfusion and BBB disruption within white matter lesions in asymptomatic subjects.
MATERIALS AND METHODS: Anatomic imaging was followed by consecutive dynamic contrast-enhanced and DSC imaging. White matter lesions in 21 asymptomatic individuals were determined using a Subject-Specific Sparse Dictionary Learning algorithm with manual correction. Perfusion-related parameters including CBF, MTT, the BBB leakage parameter, and volume transfer constant were determined.
RESULTS: MTT was significantly prolonged (7.88 [SD, 1.03] seconds) within white matter lesions compared with normal-appearing white (7.29 [SD, 1.14] seconds) and gray matter (6.67 [SD, 1.35] seconds). The volume transfer constant, measured by dynamic contrast-enhanced imaging, was significantly elevated (0.013 [SD, 0.017] minutes−1) in white matter lesions compared with normal-appearing white matter (0.007 [SD, 0.011] minutes−1). BBB disruption within white matter lesions was detected relative to normal white and gray matter using the DSC-BBB leakage parameter method so that increasing BBB disruption correlated with increasing white matter lesion volume (Spearman correlation coefficient = 0.44; P < .046).
CONCLUSIONS: A dual-contrast-injection MR imaging protocol combined with a 3D automated segmentation analysis pipeline was used to assess BBB disruption in white matter lesions on the basis of quantitative perfusion measures including the volume transfer constant (dynamic contrast-enhanced imaging), the BBB leakage parameter (DSC), and MTT (DSC). This protocol was able to detect early pathologic changes in otherwise healthy individuals.
ABBREVIATIONS:
- cSVD
- cerebrovascular small-vessel disease
- DCE
- dynamic contrast-enhanced
- Gd
- gadolinium
- K2
- BBB leakage parameter
- Ktrans
- volume transfer constant
- WML
- white matter lesion
Understanding vascular contributions that influence cognitive decline and dementia is a national research priority.1,2 Cerebrovascular small-vessel disease (cSVD) is associated with stroke and dementia and is potentially modifiable.3⇓-5 Many aspects of vascular disease of the brain can be detected with MR imaging. Features associated with cSVD include small subcortical (lacunar) infarcts, white matter hyperintensities, dilated perivascular spaces, microbleeds, brain atrophy, and increased BBB permeability.6,7 White matter lesions (WMLs) seen on T2-weighted MR imaging are the most common feature of cSVD, estimated to represent 40% of cSVD disease burden.6 WMLs are accompanied by many pathologic changes, including BBB disruption.8⇓⇓⇓⇓⇓⇓⇓⇓-17 While other multifactorial pathophysiologic mechanisms are undoubtedly involved, including hypertension, genetic factors, and inflammation,18⇓⇓⇓⇓⇓⇓-25 changes in CBF and increasing BBB permeability have been implicated as markers of WML progression and may have a causative role.13,26
Quantifying different measures of hemodynamics such as CBF, CBV, and BBB disruption directly within WMLs has been difficult. Previous studies have shown decreased CBF in larger brain regions associated with WMLs but not within WMLs themselves.6,7,17,26⇓⇓⇓⇓⇓-32 These studies have also identified increased regional nonlesional volume transfer constant (Ktrans) using gadolinium (Gd)-based dynamic contrast-enhanced (DCE) MR imaging, but these methods have drawbacks such as decreased signal discrimination within and without WMLs and a dependence on adequate correction for decreased perfusion within WMLs.7,31,33 Some studies have detected decreased CBF within lesions using arterial spin-labeling,34 while others have detected these changes within regions surrounding WMLs and within ROIs within WMLs.26,35
Presently, new imaging approaches and data-processing pipelines are needed to allow us to segment WMLs and measure subtle intralesion changes in CBF, MTT, and BBB disruption. Measures of BBB permeability incorporate MR imaging surrogates, which detect Gd extravasation outside the microvasculature due to disruption of the BBB related to microvascular injury.7,31 In the current study, on a voxel-by-voxel basis, we quantify 2 different parameters related to tissue abnormality: Ktrans from DCE MR imaging and the BBB leakage parameter (K2) from DSC MR imaging,28,29 which can relate changes in BBB transport and/or CBF.36 These values can then be assessed for WMLs to get insight into changes in BBB functioning and tissue perfusion. In addition, we assessed MTT, which reflects tissue perfusion.
In the Genetic Study of Atherosclerosis Risk (GeneSTAR) cohort study, we have identified individuals with a family history of early-onset coronary vascular disease with earlier WMLs detected in midlife,37 with a concomitant impact on measures of cognitive-motor function.38 In this relatively young high-risk subgroup (average age, 54.1 [SD, 3.5] years) of 21 participants with repeat MR imaging, we have observed rapid rates of WML progression associated with cognitive decline.39 In this study, we present a data-analysis pipeline that incorporates segmentation of WMLs40,41 and quantification of perfusion-based measures of MTT, CBF, K2, and Ktrans from both DSC and DCE MR imaging. This work builds on previous work measuring microvascular perfusion and Gd extravasation in different regions of the brain.31,42 We propose that these Gd-based representations of BBB disruption in WMLs, with knowledge of the CBF, may enable identifying WMLs at risk of progression at a stage at which they may respond to strategies of disease prevention.3,4,6
MATERIALS AND METHODS
Patient Recruitment
The 21 participants in this repeat imaging study were healthy family members of relatives with known early-onset coronary artery disease who were randomly selected (2008–2013) from a previous GeneSTAR WML study and recruited.43 The study was approved by the institutional review board and was conducted at the Johns Hopkins medical campus in Baltimore, Maryland.44 The parent study was designed to characterize the genetic and biologic traits associated with incident cardiovascular disease.38 In the larger GeneSTAR study, probands were identified during hospitalization for an acute coronary syndrome or acute myocardial infarction or with angiographic evidence of a flow-limiting stenosis before 60 years of age. Probands did not participate in the final study by design. Apparently healthy asymptomatic siblings, their offspring, and the offspring of the probands were eligible if they were 29–75 years of age and had no personal history of coronary artery disease, stroke, or TIAs. The 21 participants in this study were recruited from a cohort of 714 participants in a previous MR imaging substudy from the larger GeneSTAR study population (54.1 [SD, 3.5] years, 45% hypertensive). Written consent was obtained from each participant.
MR Imaging
The participants were examined using a 3T Achieva MR imaging scanner (Philips Healthcare) with a 32-channel head coil. The examination protocol is illustrated in the Online Supplemental Data. The structural series included axial T1-weighted MPRAGE and axial turbo spin-echo FLAIR as follows: The MPRAGE sequence was chosen over other T1WI sequences due to its excellent GM/WM contrast. The sequence parameters are the following: 1) axial T1-weighted MPRAGE: flip angle = 8°, TR = 10 ms, TE = 6 ms, voxel size = 1.0 × 1.0 × 1.0 mm, contiguous slices, FOV = 240 × 240 × 160 mm, and reconstruction matrix = 320 × 320 × 160; and 2) axial turbo spin-echo FLAIR: TR = 11,000 ms, TI = 2800 ms, TE = 68 ms, voxel size = 0.98 × 0.98 × 3.0 mm, contiguous slices, FOV = 240 × 240 × 132 mm, and reconstruction matrix = 512 × 512. The Online Supplemental Data show an example of the MPRAGE, FLAIR, precontrast baseline DCE, and DSC images from 1 participant. The structural images were reviewed by the Principal Investigator to ascertain any health concerns. The examinations were follow-up MRIs obtained after a prior MR imaging that had been reviewed by a neuroradiologist and were deemed to have normal findings with the exception of signs of WM hyperintensity and age-related microvascular disease. No lacunar infarcts were detected in this study cohort by clinical radiologists and the study investigator.
T1 mapping was performed before contrast agent injection with an inversion recovery Look-Locker sequence at resolution of 2 × 2.2 × 4 mm3 with a flip angle of 4° and a TR of 2.6 seconds. The first TI was 38.5 ms, and the spacing between successive time points was 69 ms. Thirty-eight images with different TIs were recorded for a total of 11 slices. The total scan time was 112 seconds.
A T1-weighted gradient-echo sequence was used for DCE imaging using the following parameters: flip angle = 26°, TE = 2.5 ms, TR = 5.1 ms, resolution = 22 × 2.22 × 4 mm3. Gadoteridol (ProHance; Bracco Diagnostics) was given at a dosage of 0.1 mmol/kg via a power injector. The contrast agent was injected with an injection delay of 30 seconds (14 precontrast baseline images) at a speed of 5 mL/s, followed by a 20-mL saline rinse with the same speed. The time for acquiring each dynamic scan was 2.5 seconds for 11 slices, and a total of 150 dynamic series were acquired. A postcontrast MPRAGE image was acquired after the Gd contrast injection.
Approximately 6 minutes after the DCE scan, DSC imaging was performed using single-shot EPI with TE = 29 ms, TR = 1500 ms, flip angle = 90°, and resolution = 2 × 2.2 × 4 mm.3 A second dose of gadoteridol was injected with a 15-second injection delay (10 precontrast baseline images) at a speed of 5 mL/s, followed by a 20-mL saline rinse with the same speed. The time for acquiring each dynamic scan was 1.5 seconds, and a total of 80 dynamic series were acquired for 25 slices covering most of the brain. As a direct comparison with the precontrast image, the MPRAGE sequences were repeat postcontrast, with the same parameters as those in precontrast, for direct identification of any lesion enhancement.
Data Processing
Anatomic volumes of discrete brain regions and tissue types were determined with MPRAGE images, and WML volumes were determined using FLAIR images coregistered into Montreal Neurological Institute space.45 Spatial normalization of coregistered MPRAGE and FLAIR images into Montreal Neurological Institute space was performed via affine transformation. MPRAGE images were skull-stripped and coregistered to FLAIR images. Within this pilot study, there was some enlargement of perivascular spaces observed, but they were not systematically quantified.
DCE and DSC Postprocessing
DSC, DCE dynamic images, and T1 maps were processed using nordicICE (NordicNeuroLab). For each participant, first, a precontrast T1 map was calculated by fitting the Look-Locker inversion recovery images to a model. The DCE dynamic images were motion-corrected, and the Ktrans maps were calculated using the extended Tofts model.46 The arterial input function used in the extended Tofts model was defined by taking the average of semi-automatically selected voxels (2 or 3 voxels) within the anterior cerebral arteries for each participant in the DCE images.
To calculate the CBF and MTT maps, we processed the DSC images for perfusion parameters using delay-insensitive singular value deconvolution with a threshold of 0.15 for regularization.47 The arterial input function was defined by taking the average of semi-automatically selected voxels (5 voxels) within the branches of the middle cerebral artery around the Sylvian fissure. The reason for not choosing the same arteries as in DCE is that DSC is subjected to distortions due to the EPI readout in the frontal part of the brain due to susceptibility artifacts. No leakage correction was applied for the postprocessing.
The pre-infusion images from the DCE and DSC scans were averaged and coregistered to the MPRAGE image using a fully automated pipeline, BrainMap (http://brainmap.org/software.html).48 The pipeline performed image registration using the Advanced Normalization Tools (ANTs; http://stnava.github.io/ANTs) software package,49 skull removal using Multi-cONtrast brain STRipping (MONSTR; https://www.nitrc.org/projects/monstr/),50 whole-brain gray/white matter segmentation using Multi-Atlas Cortical Reconstruction Using Implicit Surface Evolution (MaCRUISE; https://github.com/MASILab/MaCRUISE),51 and lesion segmentation using the Subject-Specific Sparse Dictionary Learning (S3DL; http://iacl.ece.jhu.edu/index.php?title=Subject_Specific_Sparse_Dictionary_Learning_for_Atlas_Based_Brain_MRI_Segmentation).41 Automatically segmented lesion masks were manually edited to remove false-positives common to this process. Segmentation masks were eroded (except for lesion masks) and applied to parameter maps to extract ROIs representing normal-appearing WM, GM, and WMLs. To extract parameter values for each participant, we took the median voxel value in each ROI from the CBF, MTT, and Ktrans maps.
In addition to calculating perfusion metrics, DSC images were also separately processed to calculate K2 values.52 DSC images are T2*-weighted, and intravascular gadolinium causes a decrease in signal due to its susceptibility artifacts. However, DSC images also have some T1-weighting that is proportional to the concentration of gadolinium in the tissue.32 During a DSC acquisition, the measured change in signal is due to both intravascular gadolinium (flow-dependent) and parenchymal gadolinium (leak-dependent).53 Using normal tissue as a reference, one can isolate the signal due to gadolinium leakage from the signal due to intravascular gadolinium.52 Normal tissue can be identified by excluding voxels that exhibit signal changes due to gadolinium leakage.28 In our case, in which leakage is small and limited to a few white matter regions, a “normal brain DSC response curve” was defined from a whole-volume analysis (about 3000 voxels). Subsequently, arrival time correction52 was applied to each individual voxel by scaling (width and height of the initial response) and shifting (position of the peak maximum of the initial response) the DSC dynamic curves. K2 in each voxel was then determined using
 in which
in which 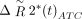 is the corrected change in relaxivity and
is the corrected change in relaxivity and 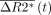 is the average signal of the normal brain. Thus, K2 reflects the proportion of the recorded signal that is due to gadolinium leakage. The resulting voxel-by-voxel measure of K2 was used to generate a blood-brain permeability image in which voxels were assigned as normal when the fitted K2 was <0.1%.
is the average signal of the normal brain. Thus, K2 reflects the proportion of the recorded signal that is due to gadolinium leakage. The resulting voxel-by-voxel measure of K2 was used to generate a blood-brain permeability image in which voxels were assigned as normal when the fitted K2 was <0.1%.
FLAIR images were coregistered to the DSC source images using a diffeomorphic registration pipeline.54 By means of the combined transforms, the WML ROIs were moved from FLAIR space to DSC space. The mean K2 value for all voxels within the WML ROIs that demonstrated elevated BBB permeability (gadolinium leakage) was calculated for each patient and used in the subsequent analysis. The K2 analysis was performed on the DSC acquisition that occurred during the second dose of gadolinium administration. K2 is largely a first-pass measure as opposed to Ktrans, which is measured in steady-state. Thus, for this study, the measured K2 reflects gadolinium leakage that occurred during the second injection and beyond steady-state background from the first injection.
Statistical Analysis
All statistical analyses were performed using R statistical and computing software (http://www.r-project.org/).55 Linear mixed effects modeling was used for statistical comparison among WM, GM, and WMLs based on the ROI level. Significance levels were assessed using paired Wilcoxon signed rank tests. Spearman correlations coefficients were calculated between lesion volumes and subjects.
RESULTS
All scans were read by the investigator and then read formally by a clinical neuroradiologist. There were no lacunar infarcts detected in this small study. Within this small group included in the pilot study, there was a significant quantity of atrophy observed and there was some enlargement of perivascular spaces observed, but these were not systematically quantified.
T1 MPRAGE images were used for GM and WM segmentation, and FLAIR images were used for lesion assignment. Notice that some lesions are not highlighted due to the size of the lesions and the detection limitations of the algorithm. The nonsegmented lesions were not included in the analysis. None of the subjects' images showed any contrast enhancement on the postcontrast MPRAGE. Two subjects were excluded due to technical problems in the contrast agent administration. Representative images for the CBF, MTT, and Ktrans maps, and their overlays on anatomic images of 1 participant are presented in the Online Supplemental Data. The perfusion images were interpolated to match the FLAIR resolution. MTT values are elevated within and around the clusters of WMLs (Online Supplemental Data). While all ROIs followed an approximately normal distribution, given the limited sample size, the median values were calculated instead of the mean. The boxplot in the Online Supplemental Data shows the median and interquartile range of MTT in the GM, WM, and WMLs of all participants. Paired Wilcoxon signed rank tests reveal that the MTT is significantly prolonged (P < .001) in WMLs (7.88 [SD, ± 1.03] seconds, median + interquartile range) compared with normal-appearing WM (6.67 [SD, 1.35] seconds), suggesting that these areas of the brain have associated vascular pathology. We also see that there is a small-but-significant difference (P < .001) in MTT for the reference WM and GM (7.29 [SD, 1.14] seconds) ROIs. CBF values were also calculated, and we found that there is no significant difference in CBF between WMLs and WM (P = .62).
The Online Supplemental Data show boxplots of the mean values for each ROI for Ktrans for all participants. Ktrans showed a much larger variance between subjects with smaller difference between ROIs. The Ktrans values were significantly different (P < .001) between the WMLs (0.013 [SD, 0.017] minutes−1) and the normal-appearing WM (0.007 [SD, 0.011] minutes−1).
The Online Supplemental Data show an example of color-coded K2 values overlaid on a gray-scale DSC source image. Voxels in the WMLs are shown in color, with increasing BBB disruption going from green (least severe) to yellow to orange to red (most severe). The Online Supplemental Data show the signal change (ΔR) with time (at each dynamic) of the recorded signal (red dashed lines) and the normal average signal (solid blue lines) before and after applying the arrival time correction. The Online Supplemental Data are for a voxel with a K2 of 0.1% (a green voxel from panel A). The Online Supplemental Data are for a voxel with a K2 value of 3.5% (a red voxel in panel A). Note that in the setting of BBB disruption, the dashed line is pulled down below the baseline due to the T1 effects from contrast leakage into the tissue. Using the DSC-K2 technique, we averaged voxels within segmented WMLs with elevated K2. The average K2 value across the cohort was 2.67 [SD, 2.33%]. This value was correlated with 3 variables: total WML volume, subcortical WML volume, and periventricular WML volume. Increasing average K2 was significantly correlated with total WML volume (Spearman correlation = 0.44; P < .046), and there was a trend toward correlation with periventricular WML volume (Spearman correlation = 0.44; P < .071). No significant correlation with subcortical WML volume (Spearman correlation = 0.0078; P < .973) was found.
DISCUSSION
The underlying pathophysiology causing cSVD and WMLs has been attributed to intermittent ischemia due to microvascular narrowing and altered compliance leading to transient hypoperfusion in vulnerable watershed zones.4,26,31 Exogenous contrast-based perfusion MR imaging has been used extensively to image hemodynamic changes in the microvasculature of ischemic white matter disease.4,27,31,32,34,35,42,56,57 Most studies have taken the strategy of quantifying perfusion parameters in different regions of the whole brain, as opposed to measuring these parameters within WMLs, and comparing them with overall WML burden.14,27,34,35,42 While a few studies have separately used arterial spin-labeling34 and Gd-based perfusion MR imaging within lesions,4,27,35,42,56 these lesion studies differ from ours in that they either used strategies focused on limited ROIs, including nonlesional white matter, or determined the WML volume using manual readers.25,27,31,54,58
Our approach is different from the approaches in these other studies in that we used an automated WML segmentation pipeline allowing us to determine the CBF, MTT, K2, and Ktrans within individual WMLs and compare them with normal-appearing WM and GM.41,45,59 Automated technology is required to study perfusion parameters and BBB disruption in large epidemiology studies to ensure consistency and speed in analysis.4,6,13 Our study design allows one to obtain perfusion parameters such as CBF, MTT, K2, and Ktrans in the same scan session. We found significant increases in MTT and Ktrans, but no significant change in CBF within WMLs compared with unaffected GM and WM. Gd leakage detected with K2 indicated more severe BBB disruption in subjects with a larger burden of WMLs.
The measurement of absolute CBF using DSC is difficult due to the lack of a direct linear relationship between contrast concentration and the signal change and partial volume effects in the arterial input function.36 Therefore, it is common to use relative values, making it difficult to make comparisons among different studies and cohorts. MTT reflects the average time for the blood to pass through a given region of brain tissue, and it is calculated by dividing the CBV by the CBF or using the Zierler area-to-height relationship.60,61 The MTT is measured in seconds, and the reverse MTT reflects the local cerebral perfusion pressure.30 MTT removes the need for obtaining absolute values of CBF and CBV; therefore, MTT has the potential to serve as a marker of hemodynamic change in white matter diseases. Tissue with decreased cellularity or metabolic activity could have decreased CBF; for example, Promjunyakul et al62 reported reduced CBF in WMLs as well as the adjacent normal-appearing WM regions beyond the WMLs, using the arterial spin-labeling technique in elderly volunteers (mean age, 84.1 years).
Our study has some differences compared with this article: First, the CBF in our article was measured using DSC MR imaging. DSC MR imaging has better contrast-to-noise in white matter than arterial spin-labeling, in which the lower SNR makes the detection of changes less sensitive.63 Also, the resolution in the above article is 3 × 3 × 4 mm3 compared with 2 × 2.2 × 4 mm3 in our study. Therefore, we could better place the voxels without the risk of partial volume effects. There should not have been a significant contribution of a perilesional zone, if any. Second, because we did not find decreased CBF within WMLs in the current study, it is not likely that the perilesional zone would have decreased the CBF. One significant difference between this article and most other literature is that the patient cohort is relatively young and asymptomatic. We found no change in CBF between WMLs and normal-appearing WM, while the MTT (CBV/CBF) was elevated, indicating compensatory vasodilation and reduced cerebrovascular resistance due to autoregulation. The elevated MTT observed in the WMLs before CBF changes may be an early indicator of asymptomatic cSVD.
Gadoteridol is a macrocyclic gadolinium-based contrast agent that is approved for multidose application. It was recommended that one-fourth-dose to 1 full-dose contrast agent preload be given 5–10 minutes before the DSC scan to reduce the T1 effect caused by contrast agent extravasation.64 In our study, one full dose of gadoteridol was injected, which served as a preload for DSC and also allowed us to perform the DCE experiment. We studied Ktrans derived from the DCE experiment using the extended Tofts model to provide more information about BBB disruption. Ktrans is a combination parameter that includes both permeability and perfusion. In a perfusion-limiting situation, the measured Ktrans may reflect perfusion instead of permeability. It has been shown that during a low-leakage situation, there is a risk that Ktrans will be overestimated using the extended Tofts model.65 Because we did not observe any visible enhancement, we believe that the risk of overestimation was avoided. We have also found that the CBF was maintained in the WMLs, and the elevated Ktrans can be attributed to increased permeability in the WMLs compared with the normal-appearing WM. However, the T1-based DCE experiment is inherently noisier, and the calculation of Ktrans requires fitting that may not be robust. In addition, Ktrans itself cannot be used to reflect permeability without knowledge of CBF.
In our study, we performed 2 gadolinium injections. DSC images are used to measure perfusion parameters. The signal change on the R2*-weighted DSC source images during the passage of a gadolinium bolus is dominated by susceptibility effects from the intravascular contrast agent. However, in the presence of gadolinium leakage, the recorded signal is augmented by a T1 effect, acting in the opposite direction, which is proportional to the concentration of gadolinium leakage (Online Supplemental Data). Thus, after arrival time correction, the effect of gadolinium leakage can be isolated as K2 (Online Supplemental Data). K2 reflects the signal change due to gadolinium leakage through the BBB during the first (and, to a lesser extent, the second) pass of the gadolinium bolus through the brain. Using the DSC-K2 approach has the advantage that the effects of blood flow and BBB permeability are being collected simultaneously during a first pass as opposing signals. In contrast, the DCE-Ktrans approach requires interpreting a separately acquired perfusion metric with a steady-state model of T1 signal change in which the effect may not be large enough to be measured in the setting of reduced blood flow. In addition, when the second gadolinium injection was given, the first injection was considered to be at steady-state. Therefore, any leakage detected by K2 for the second injection reflects first-pass effects beyond the background steady-state signal. Using the DSC-K2 method, we found that higher K2 values were significantly correlated with total WML volume, suggesting that in these asymptomatic patients, increasing WML volume is associated with increasing disruption of the BBB.
A recent article by Wong et al26 demonstrated that increased extravasation of Gd correlated with declines in CBF in the perilesional zone of WMLs. This approach solidifies the association of hypoperfusion with lesion progression and BBB disruption. The Patlak graphic method used in the article to measure Gd transition into the lesion is perfusion-dependent. Even though K2 is a dimensionless, relative measure, it has the advantage that K2 is not dependent on perfusion and is suited for detection of smaller BBB breakdown effects in regions of lower CBF. In our study, manual detection of increasing K2 was observed in proximity to WML borders as well but was not quantifiable in an automated fashion and could not be associated with declining perfusion in proximity to the WML border. Imaging techniques to simultaneously assess MTT, CBF, CBV, Ktrans, and K2 are essential to study how changes in perfusion affect WML progression14,27,31,34 and allow investigation of how subsequent BBB breakdown with associated increases in measured BBB disruptions may precede brain parenchymal injury in WMLs.4,31,42 It has been previously observed in other studies that BBB disruption in gray matter and normal-appearing white matter is increased with increasing WML burden and other signs of small-vessel disease and this has been studied extensively in white matter hyperintensities and Alzheimer disease.
Our study was not powered or designed to detect changes BBB disruption/permeability in normal-appearing white matter in general but only to compare existing WMLs and normal-appearing white matter BBB disruption using our combined imaging approach. Finding a control group without WMLs within the original study sample is very difficult because 90% of all participants in this study have signs of white matter hyperintensities, regardless of risk factors. The patients were selected across the 800 or so included in the study. They were selected for the following reasons: high or low white matter hyperintensity burden, age, sex, the presence of hypertension, and ethnicity. They were balanced to reflect the age, race, sex, and relative demographic composition of the original sample. Currently, it is difficult to know how BBB disruption could be used to predict WML progression without a study involving repeat imaging of the participant at 2 separate points in time. In future prospective studies with a larger sample size, one may have the statistical power to establish a permeability threshold above which the WMLs are at risk of expansion or the WM is at risk of lesion formation.
There are several limitations to our study. Our technique incorporated coregistration of segmented lesions of brain WMLs with brain perfusion images. The large section thickness of perfusion scans relative to the higher-resolution FLAIR images may have resulted in a partial volume effect. Additionally, some of the smaller lesions were too small to allow accurate measurement of the perfusion characteristics and, thus, were not picked up by the automatic analysis. Due to the small sizes of the lesions and the difficulty in obtaining adequate segmentation in such small areas, lesion masks were not eroded and may include some partial volume on the edges of lesions. Finally, the K2 values depend on a time integration of the difference signal for the ΔR2* curves. A disadvantage of this approach is that K2 depends on the MR imaging parameters and number of dynamics (length of the curve) used in the analysis. Thus, while the K2 values will provide an accurate reflection of BBB breakdown when used consistently within a study, they can differ in absolute value among studies.
CONCLUSIONS
The results show that MTT was increased significantly within WMLs compared with normal-appearing GM and WM, while no significant CBF alteration was found between WMLs and normal-appearing WM. These findings indicate vasodilation within the WMLs to maintain normal blood flow. Significantly elevated Ktrans was observed within WMLs compared with the normal-appearing WM. Intralesion BBB patency was also evaluated in terms of K2 using DSC data demonstrating increasing BBB disruption within WMLs with increased total WML volume. These findings suggest that DSC perfusion provides valuable information in assessing cSVD because MTT may be an early marker for an asymptomatic stage of the ischemic disease before CBF is affected and the value of BBB disruption as measured by K2 may shine light on the process of disease progression.
Footnotes
B.E. Dewey and X. Xu contributed equally to this work.
This work was supported by funding from the National Institutes of Health–National Institute of Neurological Disorders and Stroke (R01NS062059) and the Johns Hopkins Department of Anesthesia, Stimulating and Advancing ACCM Research award.
Disclosures: Linda Knutsson—RELATED: Grant: Swedish Research Council, Swedish Cancer Society*; Support for Travel to Meetings for the Study or other Purposes: Swedish Research Council, Swedish Cancer Society.* Jerry L. Prince—UNRELATED: Grants/Grants Pending: Biogen, Comments: contract on brain image processing of people with multiple sclerosis.* Peter C.M. van Zijl—UNRELATED: Grants/Grants Pending: Philips Healthcare, Comments: research, support/equipment; Payment for Lectures Including Service on Speakers Bureaus: Philips Healthcare, Comments: lectures; Patents (Planned, Pending or Issued): Philips Healthcare, Comments: patents licensed; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Philips Healthcare, Comments: travel to give lectures. *Money paid to the institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received October 16, 2020.
- Accepted after revision March 13, 2021.
- © 2021 by American Journal of Neuroradiology







