Abstract
BACKGROUND AND PURPOSE: In acute ischemic stroke, whether FLAIR vascular hyperintensities represent good or poor collaterals remains controversial. We hypothesized that extensive FLAIR vascular hyperintensities correspond to good collaterals, as indirectly assessed by the hypoperfusion intensity ratio.
MATERIALS AND METHODS: We included 244 consecutive patients eligible for reperfusion therapy with MCA stroke and pretreatment MR imaging with both FLAIR and PWI. The FLAIR vascular hyperintensity score was based on ASPECTS, ranging from 0 (no FLAIR vascular hyperintensity) to 7 (FLAIR vascular hyperintensities abutting all ASPECTS cortical areas). The hypoperfusion intensity ratio was defined as the ratio of the time-to-maximum >10-second over time-to-maximum >6-second lesion volumes. The median hypoperfusion intensity ratio was used to dichotomize good (low hypoperfusion intensity ratio) versus poor (high hypoperfusion intensity ratio) collaterals. We then studied the association between FLAIR vascular hyperintensity extent and hypoperfusion intensity ratio.
RESULTS: Hypoperfusion was present in all patients, with a median hypoperfusion intensity ratio of 0.35 (interquartile range, 0.19–0.48). The median FLAIR vascular hyperintensity score was 4 (interquartile range, 3–5). The FLAIR vascular hyperintensities were more extensive in patients with good collaterals (hypoperfusion intensity ratio ≤0.35) than with poor collaterals (hypoperfusion intensity ratio >0.35; P for Trend = .016). The FLAIR vascular hyperintensity score was independently associated with good collaterals (P for Trend = .002).
CONCLUSIONS: In patients eligible for reperfusion therapy, FLAIR vascular hyperintensity extent was associated with good collaterals, as assessed by the pretreatment hypoperfusion intensity ratio. The ASPECTS assessment of FLAIR vascular hyperintensities could be used to rapidly identify patients more likely to benefit from reperfusion therapy.
ABBREVIATIONS:
- FVH
- FLAIR vascular hyperintensity
- HIR
- hypoperfusion intensity ratio
- IQR
- interquartile range
- Tmax
- time-to-maximum
Collateral circulation plays an important role in stroke pathophysiology.1 In response to a middle cerebral artery occlusion, flow in leptomeningeal anastomoses from anterior and posterior cerebral arteries reverts and pial collaterals dilate, providing blood supply to maintain tissue viability. Assessing collateral status has direct applications for decision-making and predicting outcome after acute ischemic stroke. Using collateral-based imaging as an entry selection criterion, a recent trial showed that rapid endovascular treatment dramatically improved functional outcome in patients with proximal vessel occlusion, small infarct core, and moderate-to-good collateral circulation.2 DSA remains the reference to assess collateral status,1 but less invasive methods based on postcontrast CT2 or MR imaging3,4 have been developed. On the basis of first-pass gadolinium PWI, the hypoperfusion intensity ratio (HIR) has been creatively proposed to assess the severity of hypoperfusion.5 Defined as the proportion of the time-to-maximum (Tmax) >10-second over the Tmax >6-second lesion volumes, the HIR is a good predictor of collateral status, as demonstrated by correlations with DSA: A low HIR corresponded to good collaterals and predicted smaller infarct growth and better clinical outcome than a high HIR.5,6
A means to assess collateral status without the need for contrast agent, additional scanning time, or automated perfusion software would be desirable for patient management. FLAIR, which is part of most stroke MR imaging protocols, provides information about vessel status. High signal intensities within blood vessels, in subarachnoid spaces, distal to arterial occlusion,7 termed FLAIR vascular hyperintensities (FVHs),8,9 are related to hemodynamic impairment and represent slow retrograde flow in leptomeningeal collaterals.10 However, whether they reflect good or poor collaterals remains controversial. For some authors, FVHs represent poor collaterals and predict larger infarct growth11,12 and worse clinical outcome.12⇓–14 In the only study using the HIR to assess collaterals, extensive FVHs, assessed dichotomously as FVHs visible in >4 axial sections, were associated with high HIR (ie, poor collaterals) in 62 patients with arterial occlusion in any territory on prethrombolysis MRA.12 Conversely, other studies found that FVHs indicate good collaterals, with extensive FVHs associated with smaller baseline DWI lesions,15⇓⇓–18 less severe neurologic deficits,15⇓–17 smaller infarct growth,16,18,19 and better clinical outcome.15,18 We previously reported similar findings based on FVH presence beyond the boundaries of the cortical DWI lesion in patients with proximal MCA occlusion,9 which also predicted a better clinical response to recanalization.20 This accumulated evidence indirectly suggests that FVHs represent good collaterals. Accordingly, we aimed to determine whether extensive FVHs are associated with good collaterals, using HIR as a surrogate marker for collaterals, in a large cohort of patients with MCA territory stroke eligible for reperfusion therapy.
Materials and Methods
This retrospective study was based on a prospectively collected monocentric register of consecutive (2003–2015) patients treated by intravenous thrombolysis and/or thrombectomy. The inclusion criteria were acute ischemia in the MCA territory; pretreatment MR imaging with DWI, FLAIR, and PWI; and 24-hour follow-up MR imaging to assess infarct growth. Patients with anterior cerebral artery or posterior circulation strokes were excluded because the ASPECTS was originally designed for the MCA territory. Patients with severe artifacts on DWI, FLAIR, or PWI were also excluded. Early neurologic improvement was defined as a ≥8-point decrease in the NIHSS score within the first 24 hours or an NIHSS score ≤1 at 24 hours. Excellent or favorable outcomes were defined as 3-month mRS ≤ 1 or ≤ 2, respectively. In accordance with French legislation, ethics committee approval was not required because our study only implied retrospective analysis of anonymized data collected as part of routine clinical care.
MR imaging has been systematically implemented in our center as first-line diagnostic imaging in candidates for reperfusion therapies. Pretreatment and 24-hour follow-up MR imaging were performed on a 1.5T scanner (Signa; GE Healthcare, Milwaukee, Wisconsin) using a standardized protocol,9 including DWI, FLAIR, T2*, intracranial 3D-TOF MRA, and an additional PWI sequence for pretreatment MR imaging. FLAIR parameters were TR/TE/TI, 8277–9802/155.5–159.4/2093–2300 ms; FOV, 24 × 24 cm2; matrix, 256 × 192; 1 excitation; 24 contiguous axial sections, 6-mm thick; 2-minute18-second maximal duration. PWI consisted of a T2*-weighted echo-planar sequence with TR/TE, 2000/60 ms; FOV, 24 × 24 cm2; matrix, 64 × 96; 1 excitation; repetition, 50 times after a bolus (5–7 mL/s) of 20 mL of gadoteric acid.
FVHs were defined as focal, tubular, or serpentine hyperintensities in subarachnoid spaces with a typical arterial course.8 Blinded to PWI and clinical data, we semiquantitatively assessed FVHs using the FVH score, according to their spatial distribution in the 7 ASPECTS cortical areas (insula, M1–M6).9 An ASPECTS cortical area was considered positive when it coincided with an FVH. The FVH score ranged from 0 (no FVH) to 7 (FVHs abutting all ASPECTS cortical areas). The FVH score was additionally rated by a second reader in half of the population.
BrainStat arterial input function software (READY View; GE Healthcare) was used to generate Tmax maps. An automated 3D rigid registration (FMRIB Linear Image Registration Tool, FLIRT, Version 5.5; http://www.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT) between Tmax maps and DWI was performed and manually corrected whenever necessary. MANGO software (Version 3.8; Research Imaging Institute, UTHSCSA; http://ric.uthscsa.edu/mango/) was used to successively extract a brain mask of ADC < 1.3 × 10−3mm2/s to remove CSF voxels, project it onto Tmax maps, and extract brain voxels with Tmax >6 seconds and >10 seconds. HIR was computed as the ratio between the Tmax >10-second over Tmax >6-second volumes. Pretreatment (DWI1) and follow-up (DWI2) volumes were manually segmented using interactive tools based on DWI signal intensity.21 Infarct growth was defined as the difference between DWI2 and DWI1 volumes. A PWI-DWI mismatch was considered present when Tmax >6-second volume exceeded 1.8 × DWI1 volume.6 The occlusion site was categorized into proximal (internal carotid artery and/or M1 segment of the MCA) or distal. Complete recanalization was defined as an arterial occlusive lesion score of 3 on 24-hour MRA.
The intraclass correlation coefficient was used to assess interobserver agreement for the FVH score. For further analyses, the FVH score was considered in 4 categories (ASPECTS = 0–1, 2–3, 4–5, or 6–7) to avoid small numbers in extreme FVH scores. The median HIR in our cohort was used to dichotomize good (low HIR) versus poor (high HIR) collaterals.6,12 A univariable analysis comparing patients with low and high HIRs was used to test our hypothesis that extensive FVHs represent good collaterals (Student t or Mann-Whitney U test for continuous variables; χ2 or the Fisher exact test for categoric variables, as appropriate; and the Cochran-Armitage test for ordinal variables). Considering potential collinearity, baseline variables with a P value < .10 in the univariable analysis were entered into a multivariable binary logistic regression model with a dichotomized HIR as the dependent variable. All the above analyses were repeated using the median HIR threshold of 0.4, derived from the Diffusion Weighted Imaging Evaluation for Understanding Stroke Evolution 2 (DEFUSE 2) cohort and found to be a good predictor of collateral status.6 In an additional univariable analysis, we further compared patients according to FVH extent for clinical and imaging data, 24-hour infarct growth, early neurologic improvement, and 3-month mRS (χ2, Mann-Whitney U, or Jonckheere-Terpstra test, as appropriate). A P value < .05 was considered significant (SPSS, Version 19, IBM, Armonk, New York; SAS 9.4, SAS Institute, Cary, North Carolina).
Results
During the study period, 777 patients underwent intravenous thrombolysis and/or thrombectomy; 533 patients were excluded (pretreatment CT scan, n = 85; pretreatment MR imaging not available in DICOM format, n = 49; PWI not performed, n = 272; severe artifacts on DWI, FLAIR, or PWI, n = 26; non-MCA stroke, n = 84; no follow-up MR imaging, n = 14; or pretreatment 3T MR imaging, n = 3). They did not differ from the 244 included patients as to sex (P = .45), age (P = .46), or baseline NIHSS scores (P = .19). Two-hundred eight (85.3%) patients were treated by intravenous thrombolysis; 23 (9.4%), by thrombectomy; and 13 (5.3%), by bridging therapy. Median time to treatment was 150 minutes (interquartile range [IQR], 120–189) for thrombolysis and 230 minutes (IQR, 149–401) for thrombectomy. One hundred sixty-one (66%) patients had a proximal occlusion (internal carotid artery, n = 47, or M1, n = 114).
Interobserver agreement for the FVH score was excellent (intraclass correlation coefficient, 0.88; 95% CI, 0.84–0.91). The median FVH score in the whole population was 4 (IQR, 3–5). FVHs were abutting the insula, M2, M3, M5, M1, M6, and M4 ASPECTS areas in 240 (98%), 209 (86%), 156 (64%), 150 (61%), 126 (52%), 125 (51%), and 59 (24%) patients. Hypoperfusion was present in all patients with median Tmax >6-second and >10-second volumes of 74 mL (minimum, 0.95; maximum, 258) and 24 mL (minimum, 0.06; maximum, 160), respectively. The median HIR was 0.35 (IQR, 0.19–0.48).
In univariable analysis (Table 1), patients with good collaterals (HIR ≤ 0.35, Fig 1) had higher FVH scores (P for Trend = .016) than those with poor collaterals (HIR > 0.35, Fig 2). Patients with good collaterals also had lower baseline NIHSS scores, smaller DWI1 volumes, smaller Tmax >10-second and Tmax >6-second volumes, and more frequently had a PWI-DWI mismatch (P < .001). In multivariable analysis (Table 2), the FVH score remained significantly associated with a low HIR (P for Trend = .002), after adjustment of potential confounders. Results were similar using the alternative HIR threshold of 0.46,22 for dichotomization between good and poor collaterals (data not shown).
Comparison of patients with low and high HIRa
Factors associated with a low HIR in multivariable analysis
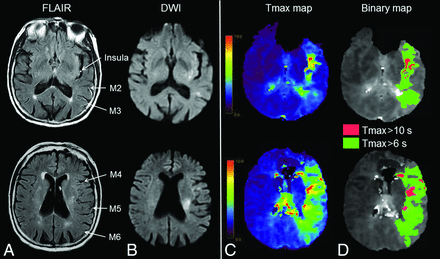
Extensive FVHs and low hypoperfusion intensity ratio. MR imaging obtained 110 minutes after stroke onset (NIHSS score = 12) in an 88-year-old woman. A, FVHs in 6 ASPECTS areas (insula, M2–M6), corresponding to a 6-point FVH-score. B, Thirteen-milliliter diffusion-weighted imaging lesion. Tmax map (C) with 85-mL Tmax >6-second lesion and 15-mL Tmax >10-second lesion (D) (HIR = 0.18). After intravenous thrombolysis, the 24-hour NIHSS score was 1, and the DWI lesion was 12 mL (not shown). The 3-month mRS was 1.
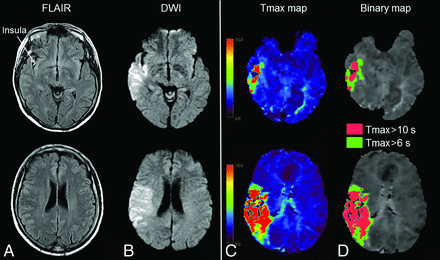
Few FVHs and high hypoperfusion intensity ratio. MR imaging obtained 180 minutes after stroke onset (NIHSS score = 12) in a 56-year-old man. A, FVHs in the insula, corresponding to a 1-point FVH score. B, Ninety-four milliliter diffusion-weighted imaging lesion. Tmax map (C) with 91-mL Tmax >6-second lesion and 53-mL Tmax >10-second lesion (D) (HIR = 0.58). After intravenous thrombolysis, the 24-hour NIHSS score was 14, and the DWI lesion was 150 mL (not shown). The 3-month mRS was 3.
When we compared patients according to their FVH scores (Table 3), patients with extensive FVHs were imaged earlier (P = .007) and had more proximal occlusions (P < .001), more PWI-DWI mismatch (P = .011), and lower HIR, considered as a continuous variable (P = .049). Despite similar baseline NIHSS scores and recanalization rates, patients with extensive FVHs tended to have smaller infarct growth (P = .125) and more frequently experienced an early neurologic improvement (P < .001) or an excellent 3-month mRS (P = .039) than those with fewer FVHs.
Comparison of patients according to FVH scorea
To rule out the potential influence of the occlusion site on our main findings, we analyzed post hoc the subset of 161 proximal occlusions, with similar results in univariable analysis (On-line Table), and again found an independent association between a high FVH score and a low HIR in multivariable analysis (P for Trend = .031). Of note, when we compared patients with proximal occlusion according to their FVH scores, patients with extensive FVHs had smaller DWI volumes (P = .003) and higher DWI-ASPECTS scores (P = .006) on pretreatment MR imaging.
Discussion
In a large population of patients with MCA stroke who underwent MR imaging with PWI before reperfusion therapy, we found the following: 1) FVH extent was independently associated with a low HIR (ie, proportionally milder hypoperfusion and hence good collaterals); and 2) patients with extensive FVHs more frequently experienced early neurologic improvement and excellent 3-month outcome, despite similar initial stroke severity and recanalization rates. These findings strengthen the notion that extensive FVHs represent a reliable surrogate for good collaterals, which maintain the viability of the ischemic tissue while awaiting reperfusion.
The presence of FVHs is considered a reliable marker of arterial occlusion7,13,23⇓–25 and, consequently, is associated with more severe admission neurologic deficits than its absence. Like others,17 we found FVHs in most patients (99%) with arterial occlusion. Besides their dichotomous presence or absence, FVH extent has been quantitated with various methods. In one method, FVHs were rated as involving less than or more than one-third of the MCA territory or the hypoperfused area,16,19 but determining this cutoff is notoriously difficult. FVHs have also been graded according to their sulcal location26 or by counting their number,11 which is cumbersome in the hyperacute clinical setting. Finally, counting axial FLAIR sections with FVHs has recently been proposed,12 but this does not account for the actual extent within each section and depends on the section number and thickness. We, like others, have previously assessed FVH topography relative to the DWI lesion, considering their rostrocaudal and anteroposterior distributions, but this approach depends on the size of the DWI lesion and does not yield the total extent of all FVHs.9,17,20 Our simple approach overcomes most of these limitations, affords an excellent interobserver concordance, and improves feasibility relative to manually counting all FVHs for clinical implementation.
Only a few studies have focused on the significance of FVH extent in the early time window. One study did not find an association between FVH extent and clinical outcome,25 while several others reported that extensive FVHs were associated with smaller baseline DWI lesions,9,15⇓–17 larger PWI-DWI mismatch,9,16 smaller infarct growth,16,18,19 and better clinical outcome.15,17,18 Similarly, we showed an independent association between extensive FVHs and a low HIR (ie, good collaterals). Patients with extensive FVHs also had a larger PWI-DWI mismatch, suggesting larger penumbra due to robust collaterals protecting the penumbra from rapidly decaying while awaiting reperfusion. Accordingly, extensive FVHs were associated with smaller ischemic lesions before treatment in patients with proximal occlusion, reinforcing our hypothesis that FVHs represent good collaterals.
The benefit of FVH-ASPECTS over simple DWI-ASPECTS for the assessment of collateral status remains to be evaluated. Most important, patients with extensive FVHs more frequently experienced early neurologic improvement and excellent 3-month outcome in contrast to those with fewer FVHs, despite more proximal occlusions and similar recanalization rates. Conversely, our findings stand in apparent contradiction to other studies suggesting that FVHs represent poor collaterals.7,12,13,23,24 However, these studies either dichotomously categorized patients into the presence or absence of FVHs7,13,23 rather than analyzing FVH extent and/or were conducted in patients not eligible for thrombolysis.24 These differences might explain the discrepancy with our findings. Perhaps more surprising are the results from Kufner et al,12 who found that FVHs rather reflected poor collaterals using the HIR in patients with arterial occlusion before thrombolysis. This discrepancy might result from several differences between this study and ours. First, Kufner et al used a 3T scanner compared with 1.5T here, and the significance of FVHs may depend on magnetic field strength and sequence parameters.27 Second, we studied a larger and more homogeneous population of overall severe anterior circulation strokes, as opposed to mixed anterior and posterior milder strokes in the Kufner et al study. The unreported proportion of posterior circulation strokes might have influenced their results because the reliability of HIR as a surrogate for good-versus-poor collaterals in this stroke subtype is unknown. Third, Kufner et al dichotomized their sample according to whether FVHs were visible over at least four 5-mm thick FLAIR sections, and such a cutoff might not have equal meaning for anterior-versus-posterior circulation strokes. Fourth, they merged patients with M1, M2, P1, P2, and vertebral occlusions into a single “medium vessel occlusion” group with an unreported proportion of distal occlusions.
FVHs might, however, be a marker for proximal occlusion23 rather than an indicator of collateral flow. Distal occlusions are indeed unlikely to result in extensive FVHs, irrespective of the quality of collaterals. However, the independent association between the FVH score and the low HIR we found in the population mixing distal and proximal occlusions remained significant in the 161 patients with proximal occlusion. This strengthens the link between FVH visibility and the quality of collaterals, irrespective of the occlusion type. Finally, the definition of HIR differed between our study and that of Kufner et al12 (Tmax 10/6 seconds here versus Tmax 8/2 seconds), but the definition we used predicted rates of collateral flow, infarct growth, and clinical outcome in the DEFUSE 2 cohort.6 Further studies are needed to determine which of the above differences accounts best for the discrepancy between the 2 studies.
Direct comparisons between FVH extent and DSA are limited.10,15,26,28 Except from a seminal study in which only 8 patients underwent DSA,7 others consistently reported FVHs to be associated with good collaterals.10,15,26,28 Our results are consistent with the latter DSA-FLAIR correlations. Although the HIR is an indirect marker of collateral status,5,6 PWI is obtained within minutes of FLAIR, thereby overcoming the issue of the delay between MR imaging and DSA inherent in all MR imaging–DSA comparisons. Given the transient nature of FVHs,7 correlation between MR images obtained nearly simultaneously provides new insights into the debated significance of FVHs.
That numerous well-developed collaterals appear as extensive FVHs deserves attention. In ischemic stroke, FVHs are undoubtedly related to retrograde collateral flow, which reaches cortical areas later than anterograde flow, often during the venous angiographic phase. This sluggish flow likely explains, at least in part, FVH visibility.10 A recent study showed that FVHs were more prominent when the arterial circulation time on DSA was neither too short (>1 second) nor too long (<7.98 seconds).28 Given that prominent FVHs are observed in a wide range of intermediate time delays of retrograde collateral filling,28 FVH visibility might depend on not only flow speed but also the amount of recruitable collaterals. Extensive FVHs could reflect the recruitment of numerous well-developed collaterals from both the anterior cerebral artery and posterior cerebral arteries (which can cover the entire ischemic bed). FVHs abutting anterosuperior ASPECTS areas may correspond to leptomeningeal collaterals from the anterior cerebral artery to the MCA, and FVHs abutting posteroinferior ASPECTS areas may correspond to leptomeningeal collaterals from the posterior cerebral artery to the MCA.26
This retrospective study has several limitations. First, collateral status was indirectly based on the HIR. The validity of the dichotomization at the median to categorize into good and poor collaterals can also be questioned. Our median HIR was, however, identical to that observed in the study of Bang et al.5 We also checked that our results were not affected by using the median HIR value of 0.4 derived from the DEFUSE 2 cohort.6 Second, despite similar recanalization rates in patients with low/high HIRs or in those with few/extensive FVHs, we cannot exclude some patients having futile recanalization because the latter was assessed on the 24-hour MRA. Third, our results are based on 1.5T MR imaging data from the same manufacturer with standardized parameters. Although this ensures data homogeneity, it prevents extrapolating to other magnetic fields, coil systems, or FLAIR parameters that may influence FVH visibility.27 Fourth, we collapsed FVH data as a single score, assuming that each ASPECTS area had the same significance. However, FVHs in different ASPECTS locations may not share the same pathophysiology.15,17,24,26 Further studies are needed to determine the implications of FVH location, if any. Last, most patients were treated by thrombolysis alone, following guidelines at the time they had a stroke. Although the association between the FVH score and HIR is independent of the type of treatment, the association with outcome might differ in patients treated according to current state-of-the-art guidelines. Because we found that extensive FVHs represented good collaterals, their link with early neurologic improvement and favorable outcome could be stronger if recanalization rates increase, as expected with bridging therapy.
Conclusions
Using quantitative assessment of FVHs and the HIR as a marker of collateral status, we found a significant association between FVH extent and good collaterals in patients with MCA stroke before reperfusion therapy. Thus, to rapidly identify patients more likely to benefit from reperfusion therapy, ASPECTS assessment of FVHs might serve as a surrogate for collateral status whenever perfusion data or fast postprocessing software is not available.
References
- Received March 17, 2017.
- Accepted after revision August 24, 2017.
- © 2018 by American Journal of Neuroradiology