We read with great interest the article entitled “Endovascular Therapy of M2 Occlusion in IMS III: Role of M2 Segment Definition and Location on Clinical and Revascularization Outcomes” by Tomsick et al.1 This study is a post hoc subgroup analysis of the patients randomized in the endovascular arm of the Interventional Management of Stroke (IMS) III study who underwent a mechanical thrombectomy (MT) for an MCA M2 segment occlusion. This article provides interesting data on distal (ie, M2) occlusions treated by endovascular means. Indeed, the recent randomized controlled trials (RCTs)2⇓⇓⇓⇓–7 that showed the effectiveness of MT in acute ischemic stroke with large vessel occlusion included very few cases of M2 occlusion (Table). Consequently, the American Heart Association (AHA) guidelines,8 according to the results of RCTs, suggest that only M1 and more proximal arterial occlusions should be safely treated by MT. Scant data (only nonrandomized, retrospective, monocenter series) on the safety and effectiveness of MT in M2 occlusions are available in the literature.9⇓⇓⇓⇓⇓–15 Despite the potential interest of this paper, we would like to raise some comments on its methods.
Number of patients with M2 occlusion in the recent randomized controlled trials on MT
First, we would like to underline the fact that a subgroup analysis, as mentioned in many papers and letters,16⇓–18 is prone to bias in the statistical analysis. Consequently, the results of such post hoc analyses on small volume subgroups should be interpreted with caution.
Second, we found it very questionable to perform a post hoc analysis of a study19 that showed such a low recanalization rate, due to the use of obsolete devices like “sonography-assisted thrombolysis” (EKOS system; EKOS, Bothell, Washington) and the “Merci retriever” (Concentric Medical, Mountain View, California), in the era of stent retrievers and aspiration devices. Indeed, the overall recanalization rate of M2 occlusion in this series was only 40%. Recent monocenter retrospective studies using more recent devices showed a recanalization rate over 75%.9,13,15 Our center's experience with distal artery occlusions treated by endovascular means shows a recanalization rate of 76%.20
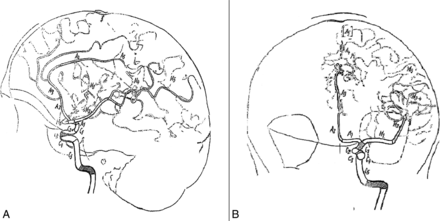
Third, we would like to report our disagreement with the MCA segmentation used in this paper. Indeed, the MCA segmentation commonly used is the one described in 1938 by Fischer21 (Fig 1) and further used in anatomic22 and angiographic23 descriptive studies. In Fischer's21 paper (written in the German language), the MCA segments are clearly defined:
Original drawings of the intracranial arteries by Fischer.21 A, Lateral view; B, frontal view. Reprinted with permission from Fischer E. Die Lageabweichungen der vorderen Hirnarterie im Gefässbild. Zentralbl Neurochir 1938;3:300–13.
Der Verlauf der A. cerebri media zerfällt in folgende Unterabschnitte:
Den horizontalen Anfangsteil (M1), von der Teilungsstelle der Carotis int. Bis zu dem etwa rechtwinkligen Knie der A. cerebri media reichend,
Den nach hinten zu ansteigenden Inselabschnitt (M2), welcher mit 2–3 Hauptästen dem Inselgebiet dicht aufliegt, im Seitenbild in der arteriellen Gefäßachse (Moniz) des Gehirns verläuft und im Vorderbild nahezu vertikal ansteigt,
Gefäßverzweigungen (M3) der vorgenannten Hauptäste der Fossa Sylvii mit dem Kandelaber (Foix) und charakteristischen Schleifenbildungen der Aa. Frontales asc. Im Seitenbild. Auf der Vorderaufnahme bilden diese zusammen mit der folgenden Gruppe ein charakteristisches, nach oben zu scharf begrenztes Fächerbild (M3–4), das bei Tumoren der Zentral- oder Parietalregion eine typische Kompression nach unten erfährt,
Gefäßverzweigungen (M4) im hintersten Teil der Fissura Sylvii (Gyrus angularis-Gebiet), die im Seitenbild deutlich hervortreten, dagegen auf der Vorderaufnahme mit dem Fächer (M3–4), zusammenfallen,
Endausbreitungen (M5) der mittleren hirnarterie, Sie sind zum Teil auf der Vorderaufnahme als feinere und mehr lockere Gefäßmaschen unmittelbar über dem dichteren und etwas gröber gezeichneten Fächerbild sichtbar, besonders klar jedoch im Seitenbild als divergiende Endäste (M5) zu erkennen (Aa. Parietalis post., angularis und temporalis post.) Bei Tumoren der Hinterhauptlappens können diese Äste von unten her eine Zusammendrängung und Parallelverlagerung nach oben oder aber, bei Entwicklung des Tumors mehr von dorsal her, eine stärkere Auseinanderdrängung in rechtwinkliger bis gerader Form erfahren.
Our translation of this article reports that:
“The course of the middle cerebral artery is decomposed in the following subsections:
The horizontal initial part (M1), from the internal carotid bifurcation to the distal genu of the middle cerebral artery.
On the lateral view, the insular section progresses along the axis of the brain arteries toward the rear and upwardly (M2) and gives birth to 2–3 main branches lying on the insula, and, on the front view, it is ascending almost vertically.
The junction of the above-mentioned main branches of the Sylvian fissure (M3) with the candelabra (Foix) shows the typical loop aspect of the ascending frontal artery on the lateral view. On the frontal view, these branches form and limit sharply with the following group a typical image of a fan turned upward (M3–4), translated downward in case of a central or parietal lobe tumor.
Vessel intersection (M4) at the rear part of the Sylvian fissure (gyrus angularis), which clearly stands out on the lateral view, whereas they coincide with the fan on the frontal view.
At the terminal section (M5) of the middle cerebral artery, there are, on the frontal view, fine and looser vascular stitches immediately above the attenuated and more visibly marked fan; however, on the lateral view, these appear particularly clear as the segments are divergent (M5) (posterior parietal, angular, and posterior temporal arteries). With occipital lobe tumors, these branches can be pushed together from downward and be translated upwardly, but with the development of more dorsal tumors, a stronger compression can shift these structures frontally.”
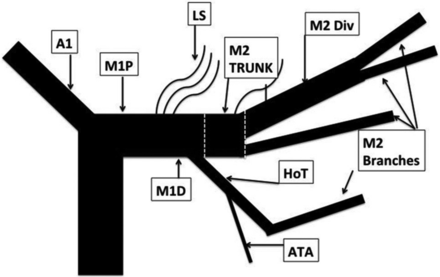
We think that using a classification without respecting criteria and landmarks that define these different segments is very confusing. Indeed, in their paper, the authors artificially created what they called an “M2 trunk” (Fig 2)1 that definitively belongs to the M1 segment according to Fischer's classification (horizontal segment, before the MCA genu). This imprecise interpretation of a segmentation commonly used worldwide may lead to substantial misunderstandings and may render the results published in this series noncomparable with other studies dealing with M2 occlusions. Recently, Goyal et al24 made an effort to clarify what should be considered as the M1 segment and detailed the M1 and M2 segment anatomic variations. In particular, they proposed to assimilate large anterior temporal artery (ATA) variation (ie, ATA supplying more than the anterior aspect of the temporal lobe) to an M2 segment.
Drawing summarizing the MCA segmentation used in Tomsick et al's1 article.
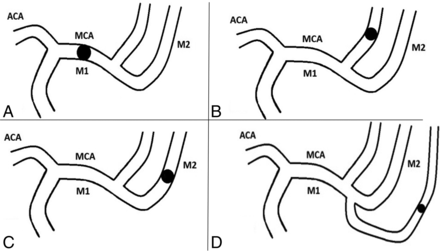
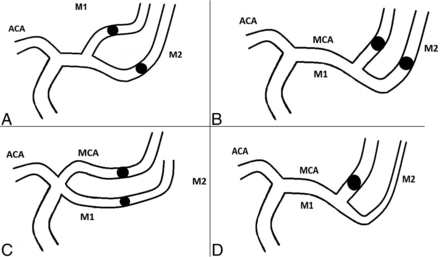
To definitively clarify what is an M1 or M2 occlusion, we suggest using a classification such as the one presented below. In this classification, in addition to true M1 or M2 occlusions (Fig 3), we describe “M1-like” (Fig 4) occlusions that comprise:
Occlusion of both branches after MCA division, proximal (short M1 segment) (Fig 4A) or distal to the MCA's genu (Fig 4B);
Occlusion of both branches of a duplicated or accessory MCA (Fig 4C); and,
Occlusion of either the superior or inferior division of the MCA, if it is a dominant branch (ie, division branch feeding ≥75% of the MCA's cortical territory) (Fig 4D).
Drawings summarizing true M1 and M2 occlusions. A, M1 occlusion; B, superior M2 occlusion; C, inferior M2 occlusion; D, M2 trifurcation occlusion. ACA indicates anterior cerebral artery.
Drawings summarizing “M1-like” occlusions. A, Occlusion of both branches after MCA division, short M1 segment; B, occlusion of both branches after MCA division, distal to the MCA's genu; C, occlusion of both branches of a duplicated or accessory MCA; D, occlusion of either the superior or inferior division of the MCA if it is a dominant branch (ie, division branch feeding ≥75% of the MCA's cortical territory). ACA indicates anterior cerebral artery.
We also describe “M2-like” (Fig 5) occlusions that comprise:
Occlusion of 1 branch after MCA division, proximal (short M1 segment) or distal to the MCA's genu (Fig 5A);
Occlusion of 1 branch of a duplicated or accessory MCA (Fig 5B); and,
Occlusion of the ATA if its trunk is large (ie, as big as M2) (Fig 5C).
Drawings summarizing “M2-like” occlusions. A, Occlusion of 1 branch after MCA division, proximal (short M1 segment) or distal to the MCA's genu; B, occlusion of 1 branch of a duplicated or accessory MCA; C, occlusion of the anterior temporal artery if its trunk is large (ie, as big as M2). ACA indicates anterior cerebral artery.
To conclude, we think that speaking the same language, by using the classifications in a common way, is the only manner to provide comparable results.
Footnotes
Disclosures: Nader Sourour—UNRELATED: Consultancy: Medtronic; Payment for Development of Educational Presentations: Medtronic, Comments: workshop; Stock/Stock Options: Medina, Comments: former investor. Bruno Bartolini—UNRELATED: Consultancy: Stryker. Frédéric Clarençon—UNRELATED: Consultancy: Codman, Medtronic.
References
- © 2017 by American Journal of Neuroradiology