Abstract
BACKGROUND AND PURPOSE: The PED is a flow-diverting stent designed for the treatment of cerebral aneurysms. We report 4 cases of delayed ipsilateral IPH following the technically successful treatment of anterior circulation aneurysms with the PED.
MATERIALS AND METHODS: Clinical and imaging data from all patients undergoing aneurysm treatment with the PED at 2 institutions were analyzed to assess the incidence of delayed IPH after treatment with the PED.
RESULTS: A total of 66 patients (47 anterior circulation) with cerebral aneurysms underwent treatment with a PED between January 2008 and November 2010. Four patients experienced delayed periprocedural IPH, all after the treatment of anterior circulation aneurysms (8.5%, 4/47). The aneurysm size ranged from 5 to 21 mm. All IPHs occurred within the cerebral hemisphere, ipsilateral to the treated aneurysm, and were anatomically remote from the treated aneurysms. All procedures were uncomplicated, and patients emerged from general anesthesia at neurologic baseline. The hemorrhages became clinically evident between 1 and 6 days after the procedure. Two patients had unfavorable outcomes (mRS scores, 4 and 6).
CONCLUSIONS: Delayed IPH may occur after the treatment of anterior circulation aneurysms with flow diverters. This complication does not seem to be restricted to a specific aneurysm subtype and does not seem to be related to an intraprocedural complication or solely attributable to DAT.
ABBREVIATIONS:
- DAT
- dual antiplatelet therapy
- FD
- flow diversion
- IPH
- intraparenchymal hemorrhage
- IQR
- interquartile range
- mRS
- modified Rankin Scale
- PED
- Pipeline Embolization Device
Flow diverters have recently received full European Conformity Mark approval for commercialization within the European Union and FDA approval for their use within the United States. To date, the reported periprocedural and midterm follow-up results have been extremely impressive,1⇓⇓⇓–5 with very high rates of complete aneurysm occlusion and relatively low rates of postoperative morbidity and mortality. However, as more experience is accrued, their potential limitations and optimal applications are becoming increasingly evident.6⇓⇓⇓⇓⇓–12
We report 4 cases of delayed ipsilateral IPH following the treatment of anterior circulation aneurysms with the PED.
Materials and Methods
Data Collection
All patients treated with the PED (ev3/Covidian, Irvine, California) between January 2008 and November 2010 at 2 different institutions were included. All available clinical and neuroimaging data were reviewed. Specifically, each case was reviewed for the occurrence of postprocedural IPH. The aneurysm size and location, procedural details, clinical presentation at the time of IPH diagnosis, subsequent imaging, and clinical outcome at last follow-up were evaluated.
PED Procedures
In general, all patients were pretreated with both aspirin (325 mg) and clopidogrel (600-mg loading dose or 75 mg daily for a minimum of 5 days) before treatment. No tests of in vitro platelet function for clopidogrel clinical response and dosing were used. One patient with hemorrhage underwent stent placement on clopidogrel only. Aspirin was introduced gradually with a desensitization protocol during the immediate postprocedural period. All procedures were performed with the patient under general anesthesia. Procedural heparinization was used to achieve a targeted activated clotting time ranging between 250 and 300s. A transfemoral access was used with either a 6F guiding catheter or 6F long sheath. Following angiographic evaluation of the targeted aneurysms, including rotational angiography, a 0.027-inch microcatheter (Marksman, ev3/Covidian) was manipulated across the targeted landing zone over a standard 0.014-inch microguidewire. Once in position, PEDs were delivered to reconstruct the parent artery defect, with multiple partially overlapping devices used as needed to bridge normal arterial segments or achieve the desired flow effect. Following stent delivery, control angiography was performed in the working angles for treatment as well as in a branch vessel projection.
Statistical Analysis
Demographic data and aneurysm size are expressed in medians and IQRs, given the small sample size.
Results
A total of 66 patients (47 anterior circulation) with cerebral aneurysms underwent treatment with the PED between January 2008 and November 2010. Of these, 4 patients experienced delayed periprocedural IPH. These included 3 females and 1 male (median age, 60 years; IQR, 15.75 years). Two patients were heavy smokers; 2 patients had well-controlled hypertension.
Aneurysm Characteristics
All hemorrhages occurred after the treatment of anterior circulation aneurysms (4 of 47 cases; 8.5%). These aneurysms involved the proximal MCA (n = 1) (Fig 1) or supraclinoid segment of the ICA (n = 3) (Figs 2⇓–4). Median aneurysm size was 9.5 mm (IQR, 4.75 mm). One of the larger aneurysms demonstrated a fusiform morphology. Three aneurysms were previously treated (1 clipped, 1 coiled, 1 PED) (Table).
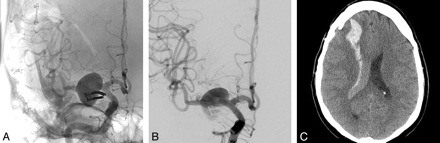
A, Right ICA anteroposterior angiogram showing a previously clipped right M1 segment aneurysm with a large recurrence. B, Immediate post-PED deployment appearance. Note the amount of contrast stasis within the aneurysm sac. C, NCCT 1 day after the procedure shows a right frontal lobar hematoma emptying into the ventricle with no significant midline shift. The patient presented with a left hemiplegia, which fully recovered on subsequent follow-up.
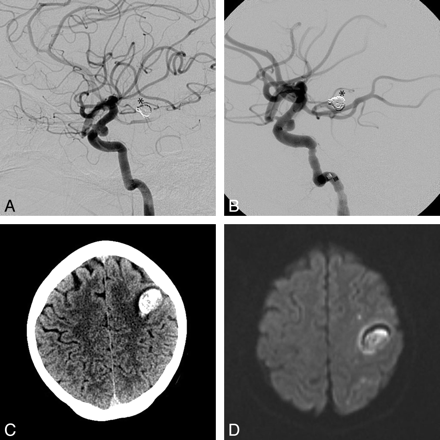
Left ICA lateral angiogram with a small broad neck supraclinoid saccular aneurysm pre- (A) and post-PED deployment (B). Coils from a previously treated basilar tip aneurysm are seen (asterisk). NCCT (C) and DWI MR imaging (D) sequences show a subcortical frontal hematoma and subcortical small ischemic lesions. Note the absence of a large associated cortical ischemic infarct.
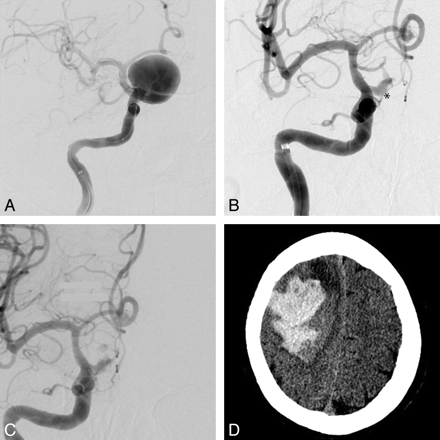
A, Initial right ICA anteroposterior (AP) angiogram with a giant supraclinoid ICA aneurysm before PED deployment (the stent is collapsed within the microcatheter). Right ICA AP angiogram 3 months later, with persistent early aneurysm filling (asterisk) pre- (B) and postdeployment (C) of 2 more PEDs. The aneurysm sac showed significant interval enlargement and development of a daughter bleb (images not shown), so a new PED was placed to increase the flow effect. D, NCCT 1 day later shows a large acute right lobar hematoma with significant mass effect. The patient survived with a persistent left attenuated hemiparesis.
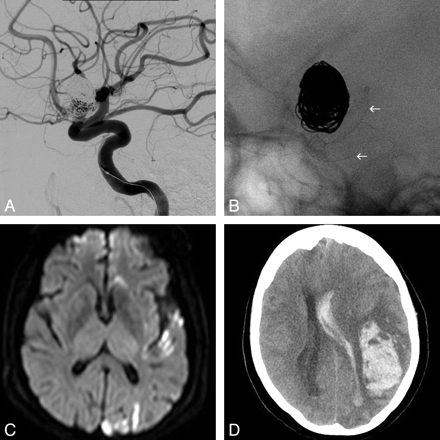
A, Left ICA lateral angiogram showing the previously coiled supraclinoid aneurysm with a significant residual filling. B, Single-shot lateral image with the PED fully deployed covering the neck of the aneurysm (arrows). C, DWI 48 hours later shows multiple left cortical ischemic lesions. D, NCCT 6 days later shows a large left frontal lobar hematoma, emptying into the ventricle, with marked midline shift and mass effect. The patient died 24 hours late.
Patients who presented with ipsilateral IPH after treatment with the PED
Intraparenchymal Hemorrhage Characteristics and Neuroimaging
All IPHs occurred within the cerebral hemisphere ipsilateral to the treated aneurysm (3 frontal lobe, 1 temporal lobe). Hemorrhages ranged in largest diameter from 36 to 72 mm (median, 59 mm; IQR, 24.75 mm). All hemorrhages were anatomically remote from the treated aneurysms. A review of the operative reports and procedural imaging by the operators did not reveal any specific intraprocedural details to which the hemorrhages could be attributed. Two of the 4 patients with hemorrhage had MR imaging–DWI either before or after the hemorrhage. In both patients, several small embolic infarcts were distributed within the ipsilateral cerebral hemisphere.
Clinical Course
All procedures were technically successful and uncomplicated. All patients emerged from general anesthesia at neurologic baseline and remained well during the immediate postoperative period. Blood pressure was closely monitored in an intensive care unit in the early postoperative period. The hemorrhages became clinically evident between 1 and 6 days after the procedure (median, 1.5 days; IQR, 2). Three patients presented with hemiplegia or hemiparesis; 1 patient presented with loss of consciousness. One patient fully recovered, 1 has slight disability, 1 has moderate/severe disability, and 1 patient died.
Discussion
Delayed Ipsilateral IPH: A Complication Related to the Treatment of Anterior Circulation Aneurysms with Flow Diversion?
Although the hemorrhages were anatomically remote from the treated aneurysms, several factors support the conclusion that these are related (in some way) to the FD procedure. First, although all procedures were technically successful and the patients emerged from anesthesia neurologically intact, all hemorrhages occurred during the immediate periprocedural (<24 hours) or early postprocedural period (days). Second, all the IPHs occurred in the vascular distribution of the reconstructed vascular segment. If a spontaneous IPH could potentially occur within either the ipsilateral hemisphere, the contralateral hemisphere, or the posterior circulation distribution, the odds of 4 consecutive IPH events occurring by chance within the ipsilateral hemisphere would be 1/3 to the fourth power or 1/81 (1.2%). Third, the rate (8.5%) at which IPH was observed in our series far exceeded that which would be expected as a sequela of dual antiplatelet therapy alone.13 For example, the annual risk of major parenchymal hemorrhage is 1.1%–1.8% in patients with DAT for secondary stroke prevention.14
The rate of IPH in this series (8.5%) is higher than the 2.2% reported after stent-assisted coiling.15 A detailed analysis of a cohort of 284 patients treated with the Neuroform stent (Boston Scientific, Natick, Massachusetts) showed a 1.1% (3/284) rate of spontaneous IPH >48 hours after treatment.16 Most interesting, the 3 delayed hemorrhages observed were equally distributed relative to the reconstructed vascular segment: 1 ipsilateral, 1 contralateral, and 1 within the posterior circulation after treatment of an anterior circulation aneurysm. These data would suggest that ipsilateral postprocedural IPH might be a phenomenon that is somehow uniquely associated with flow-diverting devices.
Delayed Ipsilateral IPH Does Not Appear to Be Related to the Size or Morphology of the Treated Lesion
The aneurysms that went on to postprocedural hemorrhage ranged widely in size, configuration, and previous treatments (Table), unlike the process of mural destabilization and delayed rupture, which appears to be almost exclusive of very large and giant aneurysms.8,10,17 As such, this complication, if consistently observed, may limit the use of the PED in aneurysms amenable for other treatment modalities with a lower overall morbidity and mortality.18⇓⇓⇓–22
Putative Etiology of Delayed Ipsilateral Parenchymal Hemorrhage after Flow Diversion
Due to the early stage of the clinical application of the FD technique and the uncharacterized but apparently low incidence of this phenomenon, the available data on which to base a mechanistic hypothesis are scant.
An initial consideration was that these hemorrhages were related to intraprocedural microwire perforations; however, this explanation was discarded for several reasons. First, the hemorrhages, though ipsilateral, were primarily lobar, distal to the aneurysms, and not consistently in a distribution that would be concordant with the intraprocedural location of the microwire. Second, all occurred at least 1 day after the index procedure, which would not be compatible with procedural vascular perforation that typically becomes clinically obvious within the first few hours.
Another consideration would be hemorrhagic transformation of clinically silent small periprocedural embolic infarcts seen in the 2 patients who had postprocedure MR imaging, as proposed by Fischer et al.23 A number of studies have quantified the incidence of small clinically silent periprocedural DWI lesions after endovascular procedures, demonstrating that such lesions are not uncommon,24⇓⇓–27 but so far, to our knowledge, no reports documenting the postprocedural hemorrhagic transformation of these types of lesions exist, even in the setting of DAT.
Our proposed hypothesis is that the reconstruction of the flexible arteries of the carotid siphon with the PED could reduce the local compliance of that vascular segment, altering the “Windkessel effect,” changing the blood pressure waveform transmitted to the distal cerebrovasculature28,29—that is, if segmental vascular reconstruction with the PED reduces arterial compliance, the blood pressure waveform transmitted beyond the reconstructed segment might exhibit a higher systolic peak and a lower diastolic trough (ie, a larger pulse pressure), and this alteration could contribute to delayed postprocedural ipsilateral IPH.30 The standard method to measure vessel wall compliance is the echo-tracking system,31 but no devices exist for intracranial vessels. Devices to measure in vivo vessel wall compliance with optical coherence elastography are under development,32 as well as noninvasive image-based computer models.33 These may allow measuring the changes in the vessel wall elasticity and comparing these changes among the different intracranial stents.
We acknowledge several limitations of our series. The sample size is small, with a heterogeneous group of aneurysm types, which precludes any statistical analysis for potential risk factors; but at the same time, this case series shows that this phenomenon is not exclusive to a specific subtype of lesion. Also, no in vitro platelet function test was used to measure the level of platelet antiaggregation, but the clinical utility in determining clopidogrel clinical response and dosing of commercially available tests is still controversial.34,35 Also, the conducted trials in the cardiology literature are designed to measure reduction in thromboembolic events.36 To our knowledge, no reports on “over-antiplatelet inhibition” and increased hemorrhagic complications have been reported in the endovascular literature.
Conclusions
Delayed ipsilateral IPH may occur after the treatment of anterior circulation aneurysms with flow diverters. This phenomenon appears independent of aneurysm size, morphology, or anatomic complexity and does not seem to be directly related to an intraprocedural event (eg, a distal wire perforation). As such, no factors have been identified that might allow operators to prospectively identify patients who might be at risk for this complication.
Footnotes
-
Paper previously presented as a Flash Presentation (3 minutes long, 5 slides) at: Annual Meeting of the World Federation of Interventional and Therapeutic Neuroradiology, Cape Town, South Africa; November 8, 2011.
-
Disclosures: Cian James O'Kelly—UNRELATED: Other: proctor fee, Comments: reimbursement for providing proctoring assistance with PED cases. David Fiorella—RELATED: Consulting Fee or Honorarium: ev3, Covidien, Comments: Pipeline proctoring (reimbursed for travel expenses only), ev3 paid consultant ($10,000), Fees for Participation in Review Activities (such as data monitoring boards, statistical analysis, end point committees, and the like): ev3, Covidien, Comments: Pipeline proctoring, UNRELATED: Consultancy: Codman Neurovascular, NFocus, WL Gore, Grants/Grants Pending: National Institutes of Health (Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis),* MicroVention/Terumo,* Siemens,* Payment for Lectures (including service on Speakers Bureaus): Codman Neurovascular, Micrus, MicroVention, ev3, Comments: Micrus-Codman-Johnson & Johnson: consulting, significant conflict ($10,000); MicroVention Terumo: consulting, significant conflict ($10,000), Patents (planned, pending, or issued): ReVasc Inc (acquired by Micrus/Codman Neurovascular), Royalties: ReVasc Inc (acquired by Micrus/Codman Neurovascular), Comments: significant conflict ($10,000), Payment for Development of Educational Presentations: Codman Neurovascular. Thomas R. Marotta—RELATED: Consulting Fee or Honorarium: proctoring for PED from ev3, Covidien, Comments: teaching other physicians how to use the device, UNRELATED: Consultancy: Evasc Medical Systems (Vancouver, British Columbia), Comments: some travel expenses, Patents (planned, pending, or issued): patent owner for eCLIPS endovascular flow diverter device. *Money paid to the institution.
References
- Received November 23, 2011.
- Accepted after revision January 25, 2012.
- © 2012 by American Journal of Neuroradiology