Abstract
BACKGROUND AND PURPOSE: In acute hepatic encephalopathy, MR imaging abnormalities have been described in the PVWM, thalami, and corticospinal tracts. We sought to determine characteristic regions of involvement on FLAIR and DWI, to evaluate their reversibility, and to correlate MR imaging extent with clinical severity.
MATERIALS AND METHODS: Twenty patients who presented clinically with acute hepatic encephalopathy and MR imaging <21 days after symptom onset were reviewed retrospectively. Two neuroradiologists recorded involved regions on FLAIR and DWI in each, measured ADC values in affected regions and NAWM, and scored the MR imaging severity/extent. The initial severity (West Haven grade), follow-up clinical severity (degree of improvement), and maximal PAL within ±8 days of MR imaging were recorded and correlated with the MR imaging severity.
RESULTS: On FLAIR and DWI respectively, there were abnormalities in the thalami (85%, 70%), PLIC (75%, 80%), PVWM (80%, 85%), and DBS (70%, 35%) and diffuse cortical involvement (30%, 25%). There were relatively strong significant (P < .005) correlations of FLAIR (r = 0.680, P = .001) and DWI severity (r = 0.690, P = .001) with PAL, and of PAL with the clinical outcome (r = 0.691, P = .001). Both FLAIR (r = 0.592, P = .006) and DWI (r = 0.487, P = .029) severity correlated moderately with the clinical outcome but were not significant at the P < .005 level after Bonferroni correction.
CONCLUSIONS: Patients with acute hepatic encephalopathy may exhibit characteristic regions of involvement on FLAIR with DWI findings that can be reversible. The MR imaging extent on FLAIR and DWI strongly correlates with the maximal PAL, and PAL correlates well with the clinical outcome. Diffuse cortical involvement has a higher potential for neurologic sequelae but can be reversible.
Abbreviations
- ACHF
- acute hepatic encephalopathy superimposed on chronic hepatic failure
- ADC
- apparent diffusion coefficient
- AHF
- acute hepatic failure
- AOD
- acetaminophen overdose
- CNS
- central nervous system
- CWM
- cerebellar white matter
- d
- days
- DBS
- dorsal brain stem
- DCI
- diffuse cortical involvement
- DWI
- diffusion-weighted imaging
- FL
- FLAIR
- FLAIR
- fluid-attenuated inversion recovery
- F-U
- follow-up
- GP
- globus pallidus
- IPH
- intraparenchymal hemorrhage
- MRI
- MR imaging
- N/A
- not available
- NAWM
- normal-appearing white matter
- nl
- normal
- NOS
- not otherwise stated
- PAL
- plasma ammonia level (maximal)
- PLIC
- posterior limb of the internal capsule
- PVWM
- periventricular white matter
- Sx
- symptom
- T1WI
- T1-weighted imaging
- T2WI
- T2-weighted imaging
Acute hepatic encephalopathy is a clinical phenomenon that is potentially reversible. The acute symptoms can be nonspecific, ranging from mild neurologic ones of altered mental status to varying degrees of unresponsiveness, with coma and death occurring in severe cases.1,2 The clinical severity has traditionally been described by the West Haven criteria (grades 1–4), with the lowest grade being mild changes in mental status and the highest grade being coma.2,3 Although the exact cause of symptoms is not entirely known, there has been a significant investigation into the mechanisms responsible for the clinical manifestations, and ammonia has been identified as a key contributor.4–7
Some have reported that the degree of hyperammonemia correlates with the degree of hepatic encephalopathy symptoms in chronic hepatic failure, while others have suggested that the same could apply in AHF.4–6 A significant but imperfect correlation between the PAL and the severity of acute hepatic encephalopathy has been noted, because there is significant overlap in PALs between different West Haven grades, while PALs may change rapidly, making it difficult to predict the clinical severity solely on the basis of the PAL.4–6 Therefore, imaging could aid in this regard, whereby the combination of characteristic MR imaging features and an MR imaging scoring system in patients with an elevated PAL could help predict the clinical severity. On presentation, patients with symptoms of acute hepatic en cephalopathy are typically evaluated by CT or MR imaging to exclude emergent phenomena such as hemorrhage or infarction; however, to date, to our knowledge, there are no definitive diagnostic imaging criteria for acute hepatic encephalopathy.
Classically, the most accepted MR imaging finding in patients with chronic hepatic failure has been hyperintensity on T1WI in the globi pallidi related to manganese, but this only variably correlates with the PAL and acute symptoms (though pallidal abnormalities have been described on DWI in acute manganese toxicity).2,8,9 Also, bilateral T1 bright signal intensity within the globi pallidi can be observed in a number of disorders that are not related to elevated manganese levels.10 Abnormalities on T2WI within the globi pallidi have been reported to accompany the T1WI findings, but these findings may be masked by T1 shortening.8,11 In a recent study, T2 (and FLAIR) hyperintense abnormalities have been demonstrated in the white matter in or around the corticospinal tracts in patients with cirrhosis, which normalized after liver transplantation.12 DWI (and ADC maps) has also identified abnormalities within the PVWM, thalami, and basal ganglia in studies of patients with cirrhosis with hepatic encephalopathy.13–16 Additionally, a report of 2 patients with ACHF with pre- and postmortem evaluation revealed abnormalities in the deep and subcortical white matter as well as cortical involvement both on T2WI and at postmortem.17 Hence, FLAIR, T2WI, or DWI MR imaging could detect such regional abnormalities in patients with acute hepatic encephalopathy from AHF or ACHF.12–16,18
Thus, because FLAIR and DWI are generally considered routine sequences for brain imaging in most institutions, these 2 sequences could be useful in determining characteristic regions of involvement. The current study was designed after noting changes on FLAIR and DWI sequences within the thalami and PLIC in 2 patients presenting with clinical symptoms of acute hepatic encephalopathy, who were part of a larger study evaluating involvement of the PVWM by toxic causes of acute leukoencephalopathy.19 Thereafter, a retrospective search of radiology reports was made for patients who presented clinically with acute hepatic encephalopathy, whether in the setting of ACHF in patients with cirrhosis or in the setting of AHF of various other causes such as acetaminophen overdose. Therefore, the goals of this study were to determine if there are characteristic regions of involvement on FLAIR or DWI in patients with acute hepatic encephalopathy, to determine the reversibility of these lesions, and to determine whether the extent of MR imaging involvement on FLAIR or DWI (MR imaging severity) would correlate with either the PAL, the initial clinical severity (West Haven criteria), or the clinical outcome severity (degree of improvement).
Materials and Methods
Patient Selection
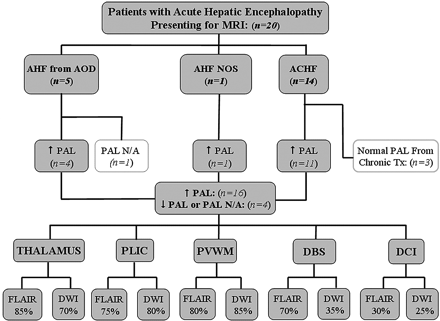
This retrospective study was approved by the internal review board. During a 2-year period (January 2007 to January 2009), 49 patients who initially presented for CT or MR imaging with symptoms of acute hepatic encephalopathy were evaluated. Thereafter, the medical records of those patients with a time between the onset of new symptoms (or of acute decline of chronic hepatic encephalopathy) to MR imaging <21 days were reviewed (n = 22) by a neurologist blinded to the imaging findings. Their medical records were reviewed by a neurologist blinded to the imaging findings. Patients were excluded for the following reasons: 1) if either no MR imaging was performed or FLAIR or DWI sequences were not performed (n = 27); 2) if the time of symptom onset was unknown (n = 1); or 3) if cerebral abnormalities were proved to occur from an etiology other than acute hepatic encephalopathy during the time they had this condition (n = 1). Notably, patients were not excluded on the basis of the location of DWI or FLAIR abnormalities because the intent was to document visually involved regions in acute hepatic encephalopathy and their frequency. Inclusion criteria were acute hepatic encephalopathy of any cause, including ACHF (n = 14); AHF from acetaminophen overdose (n = 5); or AHF not otherwise specified (n = 1) as in Fig 1. Hence, 20 patients total with a history of acute hepatic encephalopathy were included for MR imaging review. The venous PALs within ±30 days of the MR imaging were recorded, as well as the maximal PAL (if available) within ±8 days of the MR imaging (On-line Table 1).
Patients with acute hepatic encephalopathy included in this study. The number with each etiology; the number of patients with high, normal, or unavailable PALs; and the number with involvement of each region are shown.
Clinical Severity and Outcome Scoring
On-line Table 1 lists the age, sex, etiology of acute hepatic encephalopathy, clinical severity grade, maximal PAL (±8 days of MR imaging), and the clinical outcome in the included patients. The clinical severity grades at presentation were determined according to the West Haven criteria for grading mental status: 1) lack of awareness, euphoria, or shortened attention span; 2) lethargy, disorientation, or personality change; 3) somnolence to semistupor but responsive to verbal stimuli, or confusion; and 4) coma, unresponsive to verbal or noxious stimuli.2,3 A clinical-outcome severity score was then determined as the baseline cognitive performance ≥30 days after the acute event and graded from 0 to 4 on the basis of a preliminary system akin to that which has been implemented previously for grading acute toxic leukoencephalopathy: 0, complete recovery with no residual deficit; 1, mostly improved with minimal residual deficit; 2, mild residual deficit; 3, moderate residual deficit; and 4, severe outcome including no improvement, coma, or death.19 The time (in days) from symptom onset to maximal clinical improvement was also recorded (On-line Table 1).
MR Imaging Sequence Parameters
The MR imaging examinations were performed on 4 different scanners (2 at 1.5T and 2 at 3T), by using a standard protocol that included axial T1WI, T2WI, FLAIR, and DWI with ADC maps. Postcontrast T1WI was available in 12 patients. The sequence parameters for FLAIR images at 1.5T were TR/TE/TI/NEX/echo-train length, 6500–9000 ms/105–110 ms/2000–2100 ms/1–2/15–23 and, at 3T, were TR/TE/TI/NEX/echo-train length, 9000–11,000 ms/100–120 ms/2000–2100 ms/1–2/10–25. For DWI, the parameters at 1.5T were TR/TE, 3300–4000 ms/71–120 ms, and, at 3T, were TR/TE, 2800–3000 ms/70–90 ms. A gradient strength of b=1000 s/mm2 was used for DWI. The section thickness was 5 mm for each sequence.
Image Review and MR Imaging Severity Scoring
Two staff neuroradiologists (A.M.M. and J.R.B.) each with >5 years' experience in interpreting brain MR images reviewed the images jointly by consensus, blinded to the clinical symptoms or the etiology of acute hepatic encephalopathy. The selected regions on both FLAIR and DWI were tabulated as +, −, or ±; thus, ± represented probable involvement (On-line Table 2). They also graded the severity on FLAIR and DWI on the basis of an arbitrary scoring system as follows (grades 0–4, where 0 is visually normal):
Minimal (grade 1).
Symmetric involvement of ≤2 of the following: thalami, PLIC, DBS, PVWM (only 1 lobe of the cerebrum), or cerebellar white matter.
Mild (grade 2).
Symmetric involvement of ≤3 of the following: thalami, PLIC, DBS, PVWM (≤2 lobes of the cerebrum), or cerebellar white matter.
Moderate (grade 3).
Symmetric involvement of >3 of the following: thalami, PLIC, DBS, PVWM (≤3 lobes), or cerebellar white matter, but without diffuse cortical involvement.
Severe (grade 4).
Either diffuse cortical involvement or symmetric PVWM involvement of all cerebral lobes, regardless of involvement of the other structures.
The reviewers also evaluated for intracranial hemorrhage, pallidal hyperintensity on noncontrast T1WI, and parenchymal enhancement on postcontrast T1WI. They recorded the visual findings on follow-up DWI and FLAIR, available in 5 patients (On-line Table 2).
ADC Measurements
A ≥5-mm-sized region of interest was used to bilaterally measure abnormalities in the thalami, PLIC, and PVWM, with the mean of 5 measurements recorded. ADC values were not recorded in the DBS or cerebellar white matter due to potential artifacts that could yield spurious ADC values. Visually NAWM on FLAIR and DWI was used as a control ADC measurement, obtained in a similar fashion, typically from the anterior frontal deep white matter. ADC measurements were likewise performed in 5 patients who had an available follow-up MR imaging.
Statistical Analysis
A Mann-Whitney U test was used for significance in ADC values of affected areas versus NAWM and between the initial and follow-up ADC values. The Pearson correlation (r) was used to calculate correlations between each of the following: initial clinical (West Haven) grade, FLAIR severity/extent, DWI severity/extent, clinical outcome grade, and PAL. The Bonferroni correction was implemented to correct for multiple hypotheses, which reduced the significance threshold from .05 to .005, because 10 comparisons/correlations were made (Table).
Correlation of clinical severity grades of acute hepatic encephalopathy with initial MRI severity and PALa
Results
Of the 49 patients presenting with acute hepatic encephalopathy, there were 20 included for MR imaging review, consisting of 10 females and 10 males (mean age, 46.2 years; range, 10–70 years). Twenty-seven patients were excluded due to a lack of MR imaging or DWI or FLAIR sequences. We excluded 2 other patients: 1 who died within 3 weeks of MR imaging from cerebral septic emboli with multifocal abscesses simultaneously with acute hepatic encephalopathy, and the other because the date of symptom onset was unknown. The mean time of onset of new or acutely worsened symptoms to MR imaging was 6.1 days (range, 0–20 days). The time from symptom onset to the maximal clinical improvement (after which no further improvement occurred) was 13.9 days (On-line Table 1), whereas in the 12 patients in whom the neurologic status resolved (grade 0 clinically), the mean time from symptom onset to symptom resolution was 13.8 days (range, 5–27 days).
The etiologies of acute hepatic encephalopathy, the number with elevated PALs, and the percentage having each region involved on MR imaging are noted in the organizational chart (Fig 1). Of the 20 patients, 14 had ACHF and 6 had AHF (On-line Table 1). Five of the patients with AHF had acetaminophen overdose, and 1 was not otherwise specified. Of these 20 patients, 16 had an elevated PAL, and 4 had a normal or no available PAL. Notably, 3 of the 14 patients with ACHF with an available PAL had a normal PAL, but all 3 were on continual treatment that could lower the measured PAL. These 3 are of 4 patients with ACHF who were on continual therapy to reduce ammonia production (lactulose, rifaximin, or neomycin). On-line Table 1 also provides the presenting West Haven grades and the clinical-outcome grades. Four patients died with acute hepatic encephalopathy by 35 days after presentation for an MR imaging; postmortem examinations were refused by the families in each case.
On-line Table 2 describes the areas of involvement on the initial MR imaging in each patient, as well as the findings on follow-up MR imaging (n = 5). Figs 2⇓⇓–5 provide examples of regions of involvement and degrees of MR imaging severity. For the purposes of tabulating the percentages having each anatomic region involved, ± (probable involvement) was tabulated as positive. On FLAIR, thalamic involvement was present in 17/20 (85%); PLIC, in 15/20 (75%); PVWM, in 16/20 (80%); DBS, in 14/20 (70%); diffuse cortical involvement, in 6/20 (30%); and cerebellar white matter involvement, in 3/20 (15%). On DWI, thalamic involvement was present in 14/20 (70%); PLIC, in 16/20 (80%); PVWM, in 17/20 (85%); DBS, in 7/20 (35%); diffuse cortical involvement, in 5/20 (25%); and cerebellar white matter involvement, in 0/20 (0%). The ventral brain stem was involved along with the DBS on both FLAIR and DWI in 1 patient. No patient had caudate or putaminal involvement. On T1WI, bright signal intensity in the globi pallidi was noted to varying degrees in 13/20 (65%), being seen in 13/14 (93%) patients with ACHF. Hemorrhage occurred at some point in 4/20 (20%); all were subacute-phase (3 early, 1 late subacute) with mild mass effect (size range, 0.5–3 cm). None of the patients with hemorrhages were treated surgically, none had hydrocephalus, and the hemorrhage eventually resolved in each patient on serial follow-up MR imaging or CT scans. There was no parenchymal enhancement in any of the 12 patients with postcontrast T1WI available.
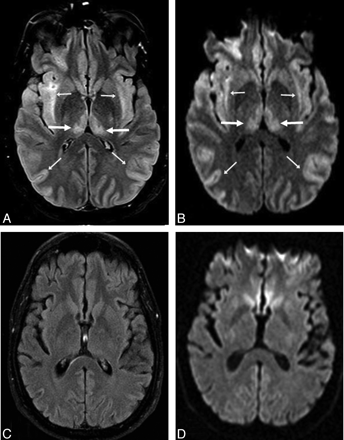
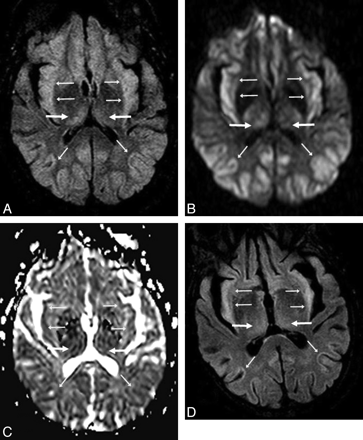
A 53-year-old man (patient 8) with acute confusion in the setting of ACHF (maximal PAL, 101 μmol/L). A−C, There is symmetric bilateral bright signal intensity in the thalami (arrows) and PLIC (thin arrows) on FLAIR (A) and DWI (B), confirmed to have reduced diffusion on ADC maps (C). D, Abnormalities are also noted in the PVWM (arrows) on FLAIR and DWI (not shown). The extent of abnormalities is graded as “moderate” on DWI and “severe” on FLAIR. The symptoms had mostly resolved 16 days later.
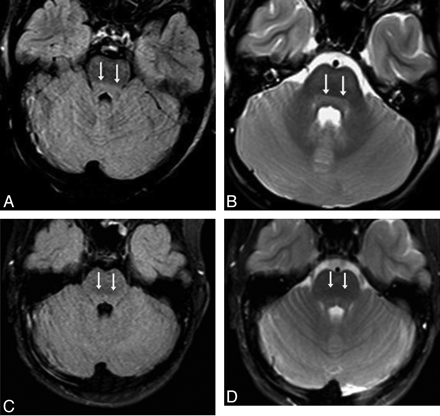
A 10-year-old girl with confusion (patient 14) from acute hepatic encephalopathy after acetaminophen overdose (maximal PAL, 104 |gmmol/L). A and B, There is predominately brain stem and thalamic involvement; the initial MR images show mild DBS hyperintensity (double arrows) on FLAIR (A) and T2WI (B). Abnormalities were also noted in the thalami (not shown); the severity was graded as “minimal” on both FLAIR and DWI (not shown). C and D, The brain stem findings have improved on MR images at 5 days, though a small frontal parenchymal hemorrhage has occurred, and soon after, the symptoms resolved completely by 13 days.
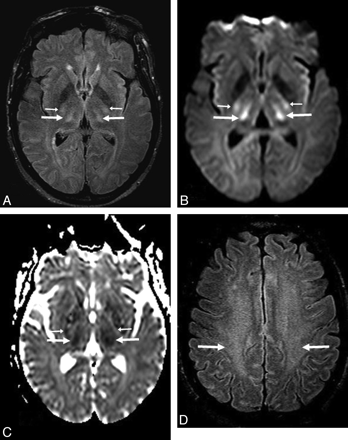
A 34-year-old confused man (patient 12) after acetaminophen overdose (maximal PAL, 206 |gmmol/L). A and B, Bilateral symmetric “severe” involvement includes abnormalities within the thalami (arrows) with diffuse cortical involvement (thin arrows), as noted on FLAIR (A) and DWI (B). C and D, On FLAIR (C) and DWI (D) 93 days later, there is complete resolution of the abnormalities, with a mild residual neurologic deficit.
A 57-year-old man (patient 9) with ACHF (maximal PAL, 223 |gmmol/L). A and B, The FLAIR (A) and DWI (B) findings are relatively symmetric, and the extent on both sequences is graded as “severe,” on the basis of involvement of the thalami (arrows) with diffuse cortical involvement (thin arrows). C, ADC maps confirm reduced diffusion extensively throughout the cortex. D, By 16 days, the diffuse cortical involvement and thalamic findings have improved on FLAIR and DWI (not shown), with residual cortical abnormalities. However, the patient died 35 days after presentation.
The Table provides correlations of each characteristic versus another with regard to West Haven grade, clinical outcome grade, maximal PAL (within ±8 days of MR imaging), FLAIR severity (extent), and DWI severity (extent). Both the FLAIR extent (r = 0.680, P = .001) and the DWI extent (r = 0.690, P = .001) had a relatively strong correlation with the PAL, and the PAL had a relatively strong correlation with the clinical outcome (r = 0.691, P = .001). There was also a strong correlation between the FLAIR extent and DWI extent (r = 0.760, P = .0001). Moderate correlations were noted between each of the following with the clinical outcome: the initial West Haven grade (r = 0.533, P = .016), the FLAIR extent (r = 0.592, P = .006), and the DWI extent (r = 0.487, P = .029). However, after Bonferroni correction for testing 10 hypotheses/comparisons (P < .05/10), these latter 3 correlations were not significant at the P < .005 level.
Regarding DWI and ADC measurements, the ADC values of each patient were recorded in the involved regions in the thalami (mean, 738.9 ± 121.2 × 10−3 mm2/s), PLIC (mean, 706.2 ± 116.5 × 10−3 mm2/s), and PVWM (mean, 729.5 ± 108.1 × 10−3 mm2/s), as well as within the NAWM (mean, 875.8 ± 126.0 × 10−3mm2/s). In patients in whom there was no visual involvement, the dorsomedial thalamus (for thalamic measurement), the posterolateral aspect of the PLIC (for PLIC measurement), and the posterior portion of the centrum semiovale (for PVWM measurement) were measured. On the initial MR imaging, ADC values were significantly decreased relative to NAWM in the thalami (P = .002), PLIC (P < .0001), and PVWM (P < .0001). On follow-up MR imaging (n = 5), the ADC values were recorded in the involved regions of the thalami (mean, 863.4 ± 79.7 × 10−3mm2/s), PLIC (mean, 791.7 ± 158.4 × 10−3mm2/s), and PVWM (mean, 792.7 ± 46.1 × 10−3mm2/s), as well as within the NAWM (mean, 856.0 ± 101.7 × 10−3mm2/s).
Follow-up ADC values were increased relative to the initial MR imaging in 4/5 patients in the thalami, 2/5 in the PLIC, and 4/5 in the PVWM, where there were no significant differences in these locations relative to the NAWM (P = .794, P = .548, P = .421, respectively). Between initial and follow-up MR imaging, the differences in mean ADC values were only significant at the P < .05 level for the thalami (P = .048), but not for the PLIC (P = .222), PVWM (P = .095), or NAWM (P = .968). On the basis of visual inspection in those patients with follow-up FLAIR available (n = 5), there was normalization in 2/5 patients who initially had thalamic abnormalities, in 1/3 patients who initially had PLIC abnormalities, in 0/2 patients who initially had PVWM abnormalities, in 1/1 patient who initially had cerebellar white matter abnormalities, in 1/4 patients who initially had brain stem abnormalities, and slow (during 3 months) normalization of the diffuse cortical involvement in 1/2 patients. On the basis of visual inspection in those patients with follow-up DWI available (n = 5), the findings normalized in 4/5 patients who initially had thalamic abnormalities (in 4/4 of those who had a follow-up MR imaging at >7 days), in 2/3 patients who initially had PLIC abnormalities, in 1/3 patients who initially had PVWM abnormalities, in 1/2 patients who initially had brain stem involvement, and in 2/2 patients who initially had diffuse cortical involvement. In the 5 patients with a follow-up MR imaging available, no parenchymal contrast enhancement was noted initially or on follow-up.
Discussion
Hepatic encephalopathy is, to date, a clinical diagnosis with a wide spectrum of manifestations. Etiologies include chronic hepatic failure with superimposed acute mental status changes (ACHF), medication-induced AHF (classically acetaminophen overdose), and other uncommon causes of AHF.20 Because acute hepatic encephalopathy is typically a clinical diagnosis, there can be reluctance in ordering MR imaging, with CT scans usually obtained to exclude emergent phenomena such as hemorrhage, herniation, or hydrocephalus. Hence, we set out to identify characteristic regions of involvement to aid the diagnosis of acute hepatic encephalopathy and found that in most patients, the thalami, PLIC, and PVWM were involved on both DWI and FLAIR. Although no specific region was involved in all patients, in our opinion, the constellation of involved areas on FLAIR and DWI is suggestive of acute hepatic encephalopathy. These findings can appear prominent but may be reversible on DWI (though less commonly on FLAIR) and are clinically reversible, as recently described by 2 reports (totaling 3 patients).19,21 Therefore, we opine that characteristic findings for acute hepatic encephalopathy on MR imaging should prompt a request for a PAL to solidify the diagnosis of this entity.
Also, while the clinical severity has been previously shown to correlate with PAL in ACHF, there can be overlapping and fluctuation of PALs with respect to different West Haven grades.4–6 Hence, we set out to determine whether MR imaging, by determining MR imaging severity, is predictive of clinical outcome. Thus, we found relatively strong correlations between MR imaging severity (based on DWI and FLAIR) with PAL and of PAL with the clinical outcome. However, the correlations between MR imaging severity and clinical outcome were only moderate and were not significant at the P < .005 level when using the fairly conservative Bonferroni correction method for multiple hypothesis testing. Hence, the direct correlation between either FLAIR severity, DWI severity, or West Haven grade with the clinical outcome is not yet entirely determined. Therefore, while acute hepatic encephalopathy can be reversible, this study suggests that the MR imaging severity may predict, to some degree, the clinical outcome of patients with acute hepatic encephalopathy, but this point should be explored further by a prospective study. Most notable is that those patients having diffuse cortical involvement are more likely to have a poor outcome, though even such diffuse insults may ultimately reverse, as noted in this study and in previous case reports.21,22
Because our study also set out to evaluate the areas of involvement and their reversibility on DWI by measuring ADC values as well, we found significantly lower ADC values on initial MR imaging in the thalami, PLIC, and PVWM relative to NAWM, but between initial and follow-up MR imaging, we found a significant difference only in the thalami. This lack of a significant difference in the PLIC and PVWM when comparing initial with follow-up MR imaging could appear contradictory. This apparent contradiction could be explained by the DWI hyperintensity noted visually in the PLIC and PVWM arising from a component of T2 bright signal intensity (T2 shinethrough phenomenon), though the significant difference in thalamic ADC suggests that the ADC values were truly decreased in that location and later normalized. However, only 5 patients underwent a repeat MR imaging, limiting statistical evaluation of this phenomenon. We note that the findings of diffuse cortical involvement improved in both patients with follow-up DWIs available, suggesting that even cases at the severe end of the spectrum acute hepatic encephalopathy can reverse.
The usefulness of FLAIR and DWI in assessing patients with hepatic encephalopathy has been illustrated in recent studies. For example, diffuse white matter hyperintensities in or around the corticospinal tracts on FLAIR or T2WI have been shown to improve posttransplantation.12 On DWI in chronic hepatic failure, several studies have found increasing ADC values in the thalami, basal ganglia, and PVWM, which have been correlated with the degree of PAL elevation and with the severity of acute hepatic encephalopathy; these findings have been proposed to represent either chronic astrocytic swelling, increased interstitial fluid, or chronic demyelination.13–16 In fulminant AHF or ACHF, recent studies have demonstrated lower ADC values in affected locations, suggesting intramyelinic edema, intracellular edema, acute astrocytic swelling, or oligodendroglial injury.23–26 Accordingly, studies using diffusion tensor imaging in patients with ACHF have recently demonstrated reduced mean diffusivity in affected regions with reduced fractional anisotropy compared with controls.24–26 Hence, we suspect that this difference in whether ADC values are increased or decreased in such involved regions would be, in part, related to the time of symptom onset, when more recent acute or early subacute lesions would demonstrate reduced ADC values due to cytotoxic effects and chronic lesions would have elevated ADC values from chronic interstitial edema or gliosis. Thus, the degree of involvement of the PLIC, thalami, and PVWM has also varied among these studies. These differences again may relate not only to the timing of MR imaging relative to symptom onset but also to the location measured (tighter white matter tracts would have lower ADC values) and possibly to the lack of visual detection of subtle findings in these regions.12–16,23–26
Future studies could use diffusion tensor imaging, MR spectroscopy, or molecular imaging to provide further insight into the mechanism of injury in acute hepatic encephalopathy, particularly by focusing on the thalami, PLIC, DBS, and PVWM. Also, a prospective evaluation of patients with acute hepatic encephalopathy via FLAIR, DWI, and ADC measurements is needed to validate our findings.
The basic physiology of acute hepatic encephalopathy involves increased levels of CNS glutamine as a result of elevated CNS ammonia levels. As CNS ammonia levels increase, glutamine, a potent osmolyte, accumulates, leading to numerous consequences such as astrocyte swelling, oxidative/nitrosative damage, disruption of glucose metabolism, defective neurotransmitter synthesis (such as gamma-aminobutyric acid), and increased blood-brain barrier permeability.7,27,28 As CNS ammonia levels rise with systemic hyperammonemia, the direct toxic effects of ammonia may take hold; thus, increasing CNS ammonia concentrations have been shown to disrupt neuronal function, and glutamine levels have been shown to correlate with the severity of hepatic encephalopathy.27–29 These changes collectively contribute to the edema and decreased consciousness that may be seen in acute hepatic encephalopathy.28–30
A few studies have simulated and studied the mechanism of abnormalities visualized on MR imaging and other imaging modalities in ammonia-related injury. For example, an experiment injecting rats with ammonium acetate noted ADC abnormalities in various regions, such as the thalami, caudate, putamen, and cerebellum, to name a few.18 On positron-emission tomography, increased blood flow and nitrogen-13 ammonia uptake have been noted in the thalami, lentiform nucleus, and cerebellum in acute hepatic encephalopathy.31 Notably, PALs have been shown to positively correlate with ADC values in the thalami, pallidi, putamen, and PVWM in patients with chronic hepatic failure.13 Hence, prior evidence along with our study suggests that a higher PAL predisposes to cytotoxic injury, which could be detected at an early stage with DWI. Therefore, the relatively strong significant correlations noted in this study between the PAL and the MR imaging severity/extent (based on FLAIR and DWI) as well as between the PAL and the clinical outcome would support the notion that the extent of injury is proportional to the concentration of ammonia delivered.
There are several limitations of this study, including the relatively small number of patients included and the retrospective nature. A prospective study in which patients with acute hepatic encephalopathy were scanned at regular intervals would be optimal. Also, because PALs can fluctuate relatively rapidly with treatment, a low PAL may belie severe MR imaging findings, particularly if imaging is relatively late after symptom onset or if the patient is already receiving continual ammonia-reduction therapy. Hence, we recorded the maximal PAL at ± 8 days of MR imaging, in an attempt to determine if overall higher PALs correlated with worse outcomes. Additionally, this study is limited by the use of different MR imaging scanners, preventing our use of a true control population for measuring ADC values; therefore, NAWM was used as a relative control. The use of NAWM as a reference is not optimal and is another limitation because subtle abnormalities within visually unaffected PVWM in acute hepatic encephalopathy may be detected only by using more sensitive tests, such as diffusion tensor imaging. However, early studies using diffusion tensor imaging in patients with acute hepatic encephalopathy have produced varied measurements in NAWM compared with controls.24–26 Notably, such findings have varied as to whether visually NAWM (and which locations) was abnormal, and the results are difficult to interpret, in part because whether such measurements were correlated with abnormalities visualized on T2WI, FLAIR, or DWI as determined by a neuroradiologist was not specified.24–26
Finally, because the PLIC may normally have mildly hyperintense signal intensity on FLAIR, T2WI, and DWI, and the DBS may also have a slightly greater intensity compared with the intensity ventrally (particularly on higher field strength magnets), we could have been predisposed to “overcalling” abnormalities in those locations and thereby generating false-positives. Therefore, a prospective study is needed that incorporates control patients, uses a single MR imaging scanner, obtains PALs at regular intervals, and implements advanced MR imaging applications such as diffusion tensor imaging, to confirm the findings of our study of affected regions on the basis of visual inspection.
Conclusions
This preliminary study suggests that in acute hepatic encephalopathy, there can be characteristic regions of involvement visualized on MR imaging and that both the clinical and MR imaging findings can be reversible. Both the FLAIR and DWI severity had relatively strong correlations with the PAL. However, both FLAIR and DWI only moderately correlated with clinical outcome, and those levels did not reach statistical significance after correction for multiple hypothesis testing. This study also confirmed the findings of previous studies that found a relatively strong correlation between PAL and clinical outcome. Hence, the use of FLAIR and DWI to determine characteristic regions of involvement in combination with the knowledge of an elevated PAL in the appropriate clinical setting could enable the early diagnosis, and potentially even prognosis, of patients with acute hepatic encephalopathy. However, prospective studies are necessary to confirm these preliminary results.
Acknowledgments
We thank Qi Wang, MS, who provided statistical support and consultation for this manuscript.
Footnotes
-
Indicates Editor's Choices selection

-
Paper presented in preliminary form at: Annual Meeting of the American Society of Neuroradiology, May 16–21, 2009; Vancouver, British Columbia, Canada.
Indicates article with supplemental on-line tables.
References
- Received January 18, 2010.
- Accepted after revision February 28, 2010.
- Copyright © American Society of Neuroradiology