Abstract
BACKGROUND AND PURPOSE: There is a high incidence of intracranial aneurysms of the AcomA suggesting the possibility of an anatomic risk factor. There also exists an association of termination-type aneurysms with anatomic variations of 1 anterior cerebral artery trunk (A1) as the exclusive or dominant supply to both pericallosal arteries (A2). This yields the hypotheses of aneurysm formation from straight jets of A1 blood.
MATERIALS AND METHODS: The anatomy and contrast filling of A1 and A2 segments and AcomAs were studied for a subset of cases entered into the Cerecyte Coil Trial for patients with AcomA (n = 105) and other aneurysms (n = 123) that were selected from imaging available at the Cerecyte Core Trial angiographic Core Lab. These cases were analyzed for A1 vessel dominance by measurement of the vessel diameter and dilution of angiographic contrast agent in A2s due to the differential flow source on selective angiography. A control group without aneurysms was assessed anatomically, using a large sequential CTA series (n = 159), acquired during acute stroke assessment.
RESULTS: A1 dominance configuration is strongly associated with the presence of AcomA aneurysms for patients with intracranial aneurysms (odds ratio, 17.8). This association is also present compared with the incidence of A1 dominance in the large sequential control series of patients without aneurysms undergoing CTA for other reasons (odds ratio, 7.5). Outflow dilution of selective angiographic images augments anatomic information.
CONCLUSIONS: A flow-based assessment of contrast flowing from the A1 to the A2 segments after injection pressure is superior to an A1 diameter based categorization when A1 vessel diameters are not strikingly different. The anatomic variant of asymmetric A1 configurations likely facilitates the development of AcomA aneurysms by flow stresses, providing further evidence to support the role of biophysical factors in intracranial aneurysm development.
Abbreviations
- AcomA
- anterior communicating artery
- A1
- anterior cerebral artery trunk
- A2
- pericallosal artery
- CI
- confidence interval
- CTA
- CT angiography
- DSA
- digital subtraction angiography
- ICA
- internal carotid artery
- MRA
- MR angiography
Aneurysms of the AcomA account for approximately a quarter of all intracranial aneurysms.1 This suggests a possible anatomic risk factor for aneurysm formation in this location. There is also a surprising incidence of asymmetry of anterior cerebral artery trunks (A1), with 1 side supplying both pericallosal arteries (A2). This anatomic variation is well known in patients with aneurysms of the AcomA.2,3
Anatomically, AcomA aneurysms are commonly termination type, when one A1 predominantly supplies both sides. In this configuration a straight jet of A1 blood is directed into a relatively wide-neck aneurysm with both A2s oriented perpendicular to the dominant A1 vessel. It is hypothesized that an asymmetric A1 anatomic configuration with one A1 providing all or most of the A2 flow is an important component in the development of such aneurysms, suggesting a causal relationship. Furthermore, a dominant A1 has previously been identified as a potential risk factor for both aneurysm formation and rupture.1,4
Kwak et al reported the incidence of single A1 vessel dominance in patients with AcomA aneurysms to be as high as 68% (145 of 213).2 That study, among others, defined hypoplasia as an A1 less then half the diameter of the dominant side when comparing left and right A1 segments.2,3,5 Other studies have defined hypoplasia as a greater than one-third difference between left and right A1 diameters4 or a vessel diameter of ≤1 mm.6 This multiplicity and variance of definitions make the associations identified within these studies challenging to compare. Furthermore, classifications based on quantitative measurements as opposed to proportional ones can be confounded by errors introduced within imaging systems, especially with pixel rather than millimeter measurements of DSA for the majority of the past 25 years.7 An assessment of vessel dominance based on supply factors that depend on inflow may be a more direct association of vessel dominance than one based on vessel diameter alone.
The extent to which vessel dominance influences the long-term results of endovascular packing of these aneurysms with detachable platinum coils is not well known.8–10 One study suggested that vessel dominance is not a major factor in predicting short-term treatment outcome; however, the definition of hypoplasia used in the study was not stated.5
Our institution runs the angiographic Core Lab of the Cerecyte Coil Trial,11 a randomized trial of aneurysm coiling with 500 patients, comparing bare platinum coils with Cerecyte coils. Of the image data received and assessed by August 15, 2009, at the angiographic Core Lab for the trial, there is a substantial subset of AcomA aneurysms. This subset of angiographic data provides a unique opportunity to assess the prevalence of A1 dominance within the circle of Willis primarily on the basis of flow and the dilution of contrast agent. Our goal was to investigate the incidence of A1 vessel variation within populations of patients with AcomA aneurysms, in patients with aneurysms elsewhere within the brain, and in patients without any aneurysms to determine its role as a risk factor for the presence of AcomA aneurysm.
Materials and Methods
With a successful amendment to our institutional ethics board approval, #051–2005, cases from the Cerecyte Coil Trial Core Lab data base that were available with complete data as of August 15, 2009, (n = 406) were divided into 2 groups: those with aneurysms of the AcomA region (AcomA, n = 105) and a random selection of the remaining 301 cases with aneurysms identified elsewhere and imaging of the anterior circulation available (non-AcomA, n = 123). The trial imaging data were mostly from selective catheter DSA, with some digital angiograms submitted without subtraction, though a few additionally included CTA and MRA, especially during follow-up.
A population of patients to serve as anatomic controls was created by reviewing craniocervical CTAs for all patients who underwent acute stroke assessment with head CT, CTA, and CT perfusion at our institution between January 2 and June 30, 2009 (n = 159). Any patient who underwent CTA more than once was only included once. Patient studies were excluded from this analysis if the study indication was related to trauma or hemorrhage due to the increased possibility of vasospasm as an artifact with anatomic measurements.
The presence of A1 dominance in each group was assessed for all groups, and odds ratio analysis was performed to evaluate its role as a risk factor. All statistical tests were deemed significant at a p value < 0.05.
Anatomic Configuration
Vessel diameters for all cases were measured at the vessel midpoint of the A1 and A2 segments and AcomAs bilaterally whenever shown. Measurements were taken as millimeters or pixels, depending on the available calibrations, case by case.7 Because all measurements for a given case were compared one to another as ratios, differences of real millimeter calibration were not relevant. Vessel segment hypoplasia was defined as the measured vessel diameter being equal to or less than half of the contralateral segment diameter.2 Blood flow through a vessel can be modeled mathematically by using the Pouseille equation relating flow, F; to vessel length, L; the pressure drop across the vessel, ΔP; the blood viscosity, η; and the vessel diameter, d:
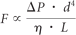
For 2 vessels of similar length and pressure difference, a 2-fold difference in vessel diameter results in a 16-fold difference in flow. Above this cutoff value, the difference in flow between the vessels very rapidly decreases. The vessel was deemed absent if no vessel was apparent at the resolution of the angiographic technique used.
The A1 to A2 flow dominance was classified on a 3-point scale: symmetric, no clear dominance of the inflow contribution of 1 A1 segment over the other; dominant, 1 A1 segment clearly contributes more inflow to an A2 than the contralateral segment; and complete, no detectable inflow contribution from the contralateral segment. Initial categorization was achieved on the basis of the vessel diameter. If the A1 segment diameters were similar, with the difference between the 2 less than half of the larger A1 diameter, they were classified initially as symmetric. Similarly if the A1 segments showed asymmetry, with the difference between the 2 being greater than half, the smaller vessel was classified as hypoplastic and the larger, as dominant. If 1 A1 segment was not apparent or was barely detectable on angiography, the case was categorized as complete.
Categorization was refined by careful inspection of the early and late dilution phases for A2 during the angiographic series. If contrast was maintained evenly in both A2 segments with injection from 1 side only, throughout those phases, the supplying A1 was deemed “dominant.” When angiography of the contralateral ICA was not submitted to the core center, vessel dominance assessment was based on the early and late dilution of A2 (n = 30, 13%). When the dilution period was not adequately captured, the initial categorization based on vessel diameter of the visible A1s was accepted (n = 23, 10%).
The vast majority (n = 203, 89%) of cases within the aneurysm groups were analyzed on the basis of DSA imaging alone. In some additional cases, supplementary analysis was used to measure and determine the anatomic configuration with MRA (n = 17, 8%) and CTA (n = 6, 3%). All cases were fully analyzed by a single observer (E.T.). A random selection of 10% of all cases was independently reviewed by a staff neuroradiologist (A.J.F.). The concurrence rate was >95%.
Results
Patient Populations
The study groups are summarized in Table 1. There were 105 patients (53 women) with AcomA aneurysms, 123 patients (92 women) with aneurysms at sites other than the anterior communicating region, and 159 patients (84 women) for the control group of CTAs performed for acute stroke. The mean age for each group was 52 ± 11 years, 50 ± 11 years, and 69 ± 15 years for AcomA, non-AcomA, and controls, respectively.
Summary of patient groups
Anatomic Configuration
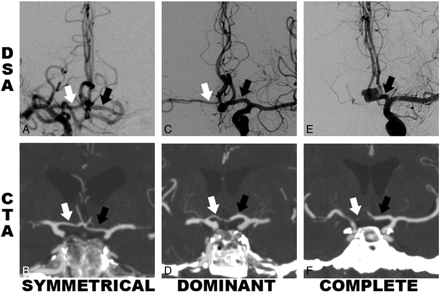
Examples of DSA and CTA images of the 3 A1 configuration categories are shown in Fig 1, with examples of symmetric, dominant, and complete filling from A1s to A2s. All DSA studies are from the Cerecyte Coil Trial, including those with AcomA aneurysms and others with aneurysms of other vessels. The same criteria were used for the CTA studies, from patients having undergone CTA for acute stroke.
Examples showing A1 segment categories with white and black arrows indicating right and left A1 segments, respectively, for the symmetric, dominant, and complete configurations. A, C, and E, DSA studies of patients with aneurysms, AcomA (C and E) and elsewhere (A). B, D, and F, CTAs used as the control group. The “symmetric” configuration from both DSA and CTA is seen in A and B, respectively. The “dominant” configuration is shown as a larger left A1 and smaller right A1 as shown in C and D, respectively. The “complete” configuration shows no evidence of the right A1 segment on E and a very small right A1 on F, with total or near-total supply to both A2s from the single A1.
Flow Configuration
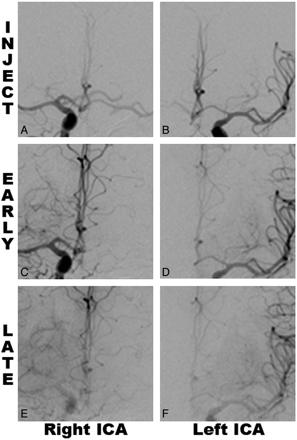
Figure 2 illustrates the concept of using the contrast injection filling and dilution phases during selective angiography to determine the A1 flow configuration. Representative still frames are shown after injection of contrast into the left ICA and right ICA in the same patient showing injection (Fig 2 A, -B), early dilution after injection pressure in the neck is removed (Fig 2 C, -D), and late dilution (Fig 2 E, -F). When we inject into the right ICA (Fig 2 A, C, and E) for this patient, both A2 segments maintain equal contrast throughout the dilution period. When we inject into the left ICA (Fig 2 B, D, and F), the contrast within both A2 segments is rapidly diluted by blood from the right A1. This exemplifies right A1 dominance with some contribution from the left A1.
A1 flow configuration on DSA from a single patient with right A1 dominance. A−F, Selective images from angiography after injection of contrast into the right ICA (A, C, and E) and left ICA (B, D, and F) for the same patient. During the pressure of injection into the ICA in the neck (A and B), contrast is seen filling both A2s from each A1. Following release of the pressure of injection, contrast continues showing the early dilution phase (C and D), maintaining both A2s filling from the right A1 (C), while there is substantial dilution of left A2 (D), injected from the left ICA. The late phase in both E and F shows all vessels as dilute. Effectively, there is a mixed supply of left A2 from both A1s, giving the classification of the configuration as “right dominant.”
The summary of A1 anatomic categorization for each study group is given in Table 2, with separate data for each category. The overall prevalence of A1 asymmetry by combining the dominant and complete categories was 69%, 11%, and 23% for patients with AcomA aneurysms, those with non-AcomA aneurysms, and stroke CTA control groups, respectively.
Anatomic configuration
In effect, there is a substantial difference in the incidence of one-sided dominance or complete supply to A2s for aneurysms of the anterior communicating region and aneurysms of other sites. Actually, a large majority of patients with AcomA aneurysms have substantial or complete supply of both A2s from a single A1. There is also a difference between the AcomA aneurysm group and the controls.
Table 3 contains the results of an odds ratio analysis. When we compared the AcomA to the non-AcomA groups, the odds of a dominant A1 configuration given the presence of an AcomA aneurysm were 14.3 times greater, with the 95% CI, 6.5–31.2. The odds of having a complete A1 configuration were 26.4 times greater, with the 95% CI, 8.9–76.8, in the AcomA aneurysm group. When the dominant and complete groups were combined, the odds were 17.8 with 95% CI, 8.9–35.3. Conversely, when we compared the AcomA group with the control group, the odds were 7.6 (95% CI, 4.0–14.6), 7.4 (95% CI, 3.6–15.0), and 7.5 (95% CI, 4.3–13.1) for the dominant, complete, and combined configurations, respectively.
Odds ratios of AcomA aneurysm presence for given anatomic configurationsa
To further assess the effect of using only anatomic vessel diameter data and a chosen vessel diameter ratio cutoff on the odds ratio, we performed a subanalysis omitting all cases without bilateral A1 vessel diameter measurements. Cases from each group were classified as dominant or symmetric on the basis of vessel diameter ratio cutoffs of 0.5, 0.6, and 0.7. The new odds ratios comparing AcomA with non-AcomA and AcomA with CTA controls for a cutoff ratio of 0.5 were 4.4 (95% CI, 2.1–9.3) and 3.8 (95% CI, 2.1–7.0). As the cutoff ratio increased, the odds ratios decreased further toward 1. Another subanalysis of AcomA aneurysms assessing dominance as a risk factor for aneurysm rupture at baseline showed an odds ratio of 1.0 (95% CI, 0.4–2.4). This implies that there is no increased incidence shown of A1 vessel dominance in the ruptured-versus-unruptured AcomA aneurysms.
Discussion
A1 dominance is an important correlate for incidence of aneurysms of the AcomA. This is in agreement with several precedent studies.2,3,6 Assessment in this current study that included flow-based analysis of dilution of contrast in the pericallosal arteries was especially useful in detecting cases of dominance in which the smaller contralateral A1 vessel still made a contribution to the flow into the A2. Specifically, 22 of 42 cases (53%) classified as dominant in this study would not have been identified by using the definition based on vessel diameter alone. Considering 40% of patients with AcomA aneurysms fell into the dominant category, this is an important factor. Most interesting, the Pouseille equation describing vessel resistance and relative flow is limited to where blood flow is laminar. Therefore, when one considers the complicated vessel branching geometry around the AcomA, the vessel diameter may become less critical in determining the subsequent flow patterns.1 This assessment of dilution of contrast from A2s during the angiographic series allows a partly physiologic method of cataloguing flow patterns, more than just the size of A1s.
Another angiographic study using a flow-based analysis found that a single A1 vessel supplied both A2s in 57% of patients with AcomA aneurysms.12 The authors' assessment of flow differed from that used in this study in that categorization was based on contrast filling 1 or both A2s from each A1 and did not take into account the outflow dilution patterns from the vessels once injection pressure was removed. The dilution of contrast due to physiologic flow used in our study is not subject to the same variation as the contrast filling phase, in which the catheter position and injection pressure will greatly influence the filling pattern and hence the categorization of these vessels.
The odds ratios in our study of A1 asymmetry (dominant or complete) in the AcomA group, 17.8 (95% CI, 8.9–35.3) and 7.5 (95% CI, 4.3–13.1), differed from those in the non-AcomA and control groups respectively, suggesting a strong association of A1 asymmetry as a risk factor for aneurysm formation. The potential explanations for the difference in the odds ratios between the non-AcomA and control groups are 2-fold. First, the additional flow dilution information for the AcomA and non-AcomA groups increased our detection of vessel asymmetry, whereas the 0.5 diameter ratio cutoff could only be used for detection with controls. Second, the incidence of A1 vessel hypoplasia was 11% in the non-AcomA group versus 23% in the CTA-control group. This difference in incidence might be attributed to the fact that the non-AcomA group did not have any AcomA aneurysms present. Therefore, this population had already been artificially selected against AcomA aneurysm formation and the anatomic configurations associated with its incidence.
Our control group was limited in that CTA fills all vessels; therefore, there was no dilution of vessels available to analyze for the relative degree of contribution of A1 to A2 as there was with the serial selective angiography, leaving the anatomic information only. A subanalysis mentioned in the “Results” section implies that the differences between the odds ratios between the groups can be accounted for by the detection method used. The decrease in the odds ratio for the 0.5 cutoff can be accounted for by the cases of dominance missed by not using flow dilution information to assist in classification. The overall decrease in the odds ratios toward 1 as the cutoff increases shows that vessel dominance becomes less of a risk factor for AcomA aneurysm incidence as the threshold for the difference between vessel diameters to classify dominance is decreased. This supports the hypothesis that the more complete the dominance of the vessel, the stronger is its role as a risk factor for aneurysm incidence.
Due to our retrospective study design, we were not able to assess the risk of developing an AcomA aneurysm given a certain anatomic configuration; however, this study analyzed the relationships between A1s in size and flow dominance to AcomA aneurysm incidence and is not a longitudinal study testing these factors in relation to future aneurysm growth.
Our overall incidence of A1 asymmetry in the AcomA group was 69%, which is in agreement with previous studies.2,12 A follow-up study looking at aneurysm development with time found that a hypoplastic vessel within the circle of Willis was present in 5 of 7 (71%) patients with aneurysm development.6
Our study was limited in a number of ways. For example, the image material available from the data subset was part of the Cerecyte Coil Trial and had been submitted under their trial protocol. The inclusion criteria selected patients between 18 and 70 years of age with ruptured or unruptured intracranial aneurysms scheduled for treatment. The aneurysm could not exceed a maximum lumen diameter of 18 mm, and the neck had to be at least 2 mm wide to be randomized into the trial. Large aneurysms and those with necks <2 mm were excluded from randomization. The inclusion of ruptured and unruptured aneurysms and angiographic vasospasm at baseline in the Cerecyte trial is a potential confounding factor for angiographic measurements performed in our current study. Angiographic vasospasm at baseline was identified in <10% of all DSA cases. For those cases, measurements of A1s and A2s were adjusted on the basis of follow-up imaging after reversal of vasospasm. A separate subanalysis of ruptured-versus-unruptured aneurysms at baseline, mentioned in the “Results,” failed to show a difference between the 2 groups in both incidence of vessel asymmetry and odds ratio analysis. Furthermore, vessel dominance was not found to be a risk factor for AcomA aneurysm rupture.
Technical variability exists in angiography for exact views and volume and rate of contrast injection as well as catheter positions and overlying A1, AcomA, and A2 vessel branches. It was not possible to determine 3D bifurcation angles13 from submitted 2D DSA studies, and a complete DSA series for all patients showing all vessels was not available.
To determine the effect of the incomplete cases on the overall data, we performed a separate odds ratio subanalysis, both omitting incomplete cases and assuming a symmetric A1 configuration in cases without contralateral ICA imaging. The results remained consistent with the initial analysis.
The assessment of flow dominance in this report only refers to dilution of A2s (pericallosal arteries), which was also limited by technical variability in the imaging data as mentioned above. Given the available case material, an opportunity to refine an anatomic definition of dominance more consistent with flow dominance was not possible as it might be for a prospective study in which imaging methodology can be held more consistent.
It seems then that anatomic variations within the anterior part of the circle of Willis should play a role in the development of aneurysms by producing differing hemodynamic stress.14 On DSA and CTA studies for these cases, we generally see that the dominant A1 flow jet is aligned in the direction of the AcomA aneurysm, with the A2 branches off to the side. There are several hypotheses as to the precise mechanisms by which this configuration predisposes to aneurysm formation, including wall shear stress, A1/A2 bifurcation angles, and flow patterns influenced by the vessel geometry.1,8,13 Perhaps all of these factors interact. The key observation lies in realizing that the stresses of hemodynamic foci of laminar and turbulent flow occur cyclically, at a frequency of approximately 60–75 times per minute every hour of every day, month, and year of one's life. The association between the high incidence of AcomA aneurysms in the presence of these anterior cerebral anatomic variants supports the relationship of cause and effect.
There is a growing use of CTA or MRA for screening for aneurysms, especially in high-risk groups.15 Our results suggest that it is possible that a higher risk of aneurysm development, growth, and rupture risk exists for A1 dominance. However, because this was not a longitudinal study of those developments, it is premature at this time to indicate routine follow-up in cases negative for aneurysm with this configuration. More investigation is needed to show future development of AcomA aneurysms in patients with anatomic risk factors for aneurysms.
Conclusions
Anatomic variants of the circle of Willis, such as A1 dominance to supply both A2s, are a strong risk factor because their incidence is high when aneurysms of the AcomA are present. A flow-based interpretative assessment focusing on dilution of contrast flowing from the A1 to the A2 segments after injection pressure is effective in detecting asymmetries and is superior to an A1 diameter−based categorization when A1 vessel diameters are not strikingly different. The association between A1 asymmetry, long-term aneurysm development, and long-term aneurysm treatment outcome should be investigated to determine whether patients with these anatomic variants would benefit from closer follow-up. This dilution is based on selective angiographic series. The challenge now remains as to how to assess a correlate of A2 dilution with CTA or MRA, as these modalities become the dominant angiographic methods of intracranial arterial assessment. The advent of 320-section CT scanners capable of imaging contrast injection and dilution suggests that a dilution-based intracranial arterial assessment may be feasible in the near future.16
Acknowledgments
We thank the organizers and supporters of the Cerecyte Coil Trial, including A.J. Molyneux, MD, as Chief Investigator; and Mary Sneade of the Oxford Neurovascular and Neuroradiology Unit, University of Oxford, United Kingdom, for coordinating the trial.
Footnotes
-
This work was supported by Sunnybrook Research Foundation through the Linda McCleod Memorial Fund and its founder Ouilla Shirriff and by Micrus Endovascular Corporation, San Jose, California, providing funding as a trial sponsor.
References
- Received November 14, 2009.
- Accepted after revision January 21, 2010.
- Copyright © American Society of Neuroradiology