Abstract
SUMMARY: The coronavirus disease 2019 (COVID-19) pandemic caused by Severe Acute Respiratory Syndrome coronavirus disease 2 (SARS CoV-2) most commonly presents with respiratory disease, but neurologic complications are being reported. We aimed to investigate the rate of positive neuroimaging findings in children positive for SARS-CoV-2 referred for neuroimaging between March 18 and September 30, 2020. We found that 10% (n = 2) had acute findings. Our results may suggest that in children, neurologic involvement in COVID-19 is rare, neuroimaging has a low yield in diagnosis, and acute neuroimaging should involve careful risk-benefit analysis.
ABBREVIATIONS:
- COVID-19
- coronavirus disease 2019
- MIS-C
- multisystem inflammatory syndrome in children
- SARS-CoV-2
- Severe Acute Respiratory Syndrome coronavirus disease 2
The coronavirus disease 2019 (COVID-19) pandemic is caused by Severe Acute Respiratory Syndrome coronavirus 2 (SARS CoV-2). The most common presentation of SARS-CoV-2 infection is respiratory disease, but associated neurologic complications are increasingly reported in adults.1
A wide spectrum of neurologic symptoms has been described. Common neurologic manifestations include fatigue, headache, and smell and taste disorders.1 In addition, the following serious neurologic complications associated with COVID-19 have been reported:2 1) cerebrovascular accidents (ischemic stroke and macro-/microhemorrhages), 2) encephalopathies, 3) infectious-/immune-mediated complications (Guillain-Barre syndrome, acute disseminated encephalomyelitis), 4) meningoencephalitis, 5) seizures, and 6) neuropsychiatric symptoms (psychosis, mood disorders).
Because COVID-19-associated neurologic manifestations or symptoms are less frequent (1.5% versus 36.4%) and usually less severe in children and, in particular, neonates, neuroimaging findings are uncommon relative to adults.3 The goal of this article was to investigate the neuroimaging findings and yield of neuroimaging in children positive for SARS-CoV-2 with suspected neurologic involvement.
MATERIALS AND METHODS
Following institutional review board approval, a master database of all patients who tested positive for SARS-CoV-2 at Texas Children’s Hospital between March 18, 2020, and September 30, 2020, was assembled by the Texas Children’s Hospital COVID-19 Imaging Taskforce. All imaging studies were extracted from the master database, and the neuroimaging studies were identified for this retrospective study. Data on demographics, new-onset neurologic symptoms, clinical features (comorbidities, respiratory symptoms, multisystem inflammatory syndrome in children [MIS-C]), cardiopulmonary support, intensive care unit or special isolation unit stay, immune-therapy and condition at discharge, and laboratory findings (CSF testing, blood testing, SARS-CoV-2 testing) were reviewed and extracted from the electronic medical records.
New-onset neurologic symptoms that were primary indications for neuroimaging within 1 month of testing positive for SARS-CoV-2 were classified as the following: 1) COVID-19-attributable indications (fever, seizures, status epilepticus, headache, focal neurologic examination findings, impaired consciousness);1⇓-3 and 2) other indications (motor vehicle crash, abusive head trauma, penetrating trauma, history of ventriculoperitoneal shunt, hydrocephalus, global developmental delay, sensorineural hearing loss, primary or metastatic tumor). Only children who met all of the following criteria were included in this study: 1) younger than 18 years, 2) tested positive for SARS-CoV-2 before and within 1 month of neuroimaging, and 3) had neuroimaging studies with COVID-19-attributable indications. The neuroimaging studies were re-evaluated for this study by 2 experienced pediatric neuroradiologists (S.F.K. and N.K.D. with 9 and 10 years of experience, respectively) in consensus.
RESULTS
The COVID-19 Imaging Taskforce identified 4351 patients, of whom 3694 were children (0–18 years of age) who tested positive for SARS-CoV-2 by polymerase chain reaction and/or serum antibodies at the Texas Children’s Hospital during the study period. There were 3364 imaging studies performed on these patients, of which 217 (6.5%) were neuroimaging studies.
Forty-three neuroimaging studies (17 head CTs, 11 without contrast, 6 with contrast; 26 brain MRIs, 8 stroke protocol, 3 with/without contrast, 6 without contrast, 2 MRVs, and 7 MRAs) of 20 children (male/female, 12:8) met our inclusion criteria. All children had at least 1 neuroimaging study, 8 children had follow-up studies within a 4.47-day interval (range, 0–72 days). The average age at neuroimaging was 8.8 years (range, 0.6–17.8 years). Fifty-five percent of patients (n = 11) had no previous medical conditions. The remaining patients had the following pre-existent conditions: epilepsy (patient 3), sickle cell disease (patients 7 and 10), obesity (patients 11 and 13), overweight (patient 17), hemophilia C (patient 14), Sturge-Weber syndrome (patient 18), and autism (patient 20) (Online Supplemental Data).
The patients’ neurologic presentation timeline was April (n = 1), June (n = 6), July (n = 6), August (n = 4), and September (n = 3) of 2020. Ten percent of patients (n = 2) had respiratory symptoms, MIS-C was noted in 10% of patients (n = 2), and 15% of patients (n = 3) had both. Mechanical ventilation was required in 2 patients, and mechanical ventilation with extracorporeal membrane oxygenation was required in 2 additional patients. Thirty percent of patients (n = 6) stayed in our special isolation unit for an average of 5.3 days (range, 1–14 days), and 25% of patients (n = 5) stayed in the intensive care unit for an average of 11.2 days (range, 2–25 days). CSF was normal in 3 patients, but no CSF records were available for the remaining 17 patients. Blood testing showed increased inflammatory markers in 55% of patients (n = 11), findings were normal in 25% of patients (n = 5), and results were not available in 20% of patients (n = 4). Thirty percent of patients (n = 6) received immune therapy, and 10% (n = 2) received antiviral therapy as part of their COVID-19 management (Online Supplemental Data). Two patients (10%) had no follow-up records, and 90% of the patients (n = 18) were discharged from the hospital with either an improved (60%) or good (30%) condition (Online Supplemental Data).
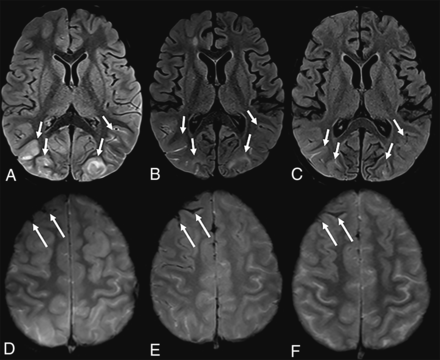
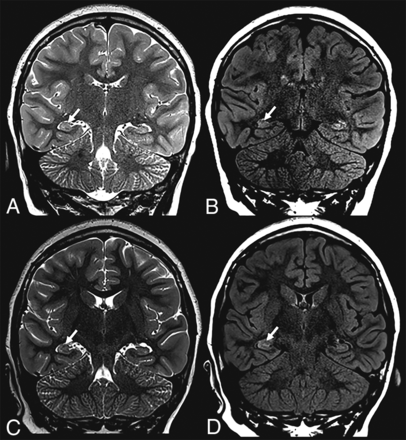
The mean time interval between SARS-CoV-2 testing and the initial neuroimaging study was 3.9 days (range, 0–22 days). Neurologic symptoms at neuroimaging included impaired consciousness (n = 7), seizures (n = 4), status epilepticus (n = 2), headache (n = 2), focal neurologic findings on examination (n = 2), fever with meningeal signs on examination (n = 1), transient episode of aphasia (n = 1), and fever with headache (n = 1). Only 2 patients (10%) had acute findings on their initial MR imaging studies: subarachnoid hemorrhage combined with posterior reversible encephalopathy syndrome in patient 7 (Fig 1) and a right-sided hippocampal T2-hyperintense signal alteration in patient 19 (Fig 2), possibly secondary to seizure activity. Of the 5 children diagnosed with MIS-C, only 1 patient (patient 19) had acute imaging findings (right hippocampal edema).
A 7-year-old boy with sickle cell disease who presented with dyspnea and chest pain tested positive for SARS-CoV-2. The patient was unresponsive, having desaturations and being intubated. Brain MR imaging showed T2-FLAIR hyperintensity and cortical edema in the occipital lobes, consistent with posterior reversible encephalopathy syndrome, partially resolving on subsequent imaging (A–C, arrows). Note interval evolution of right frontal subarachnoid hemorrhage (D–F, arrows).
A previously healthy 9-year-old girl who presented with status epilepticus tested positive for SARS-CoV-2. Brain MR imaging showed subtle right hippocampal T2-FLAIR signal alteration with corresponding edema (arrows) on initial (upper row) and follow-up (lower row) imaging.
DISCUSSION
In this limited, preliminary study, we demonstrated systemic and neurologic manifestations and neuroimaging findings in 20 children positive for SARS-CoV-2. We found that 10% of patients (n = 2) had acute findings on their neuroimaging studies; in 90% of patients, neuroimaging did not show acute pathology that could be attributed to the SARS-CoV-2 infection.
SARS-CoV-2 neurotropism is still poorly understood, but 4 potential mechanisms have been proposed to explain COVID-19 neurologic involvement: 1) a secondary effect of the systemic inflammatory responses triggered by the viral infection; 2) a secondary effect associated with the vascular and prothrombotic effect of the viral infection on the nervous system vasculature; 3) an immune-mediated parainfectious or postinfectious autoimmune effect in response to the viral infection; and 4) a direct neurotropic or neuroinvasive effect of SARS-CoV-2. Direct viral invasion confirmation would require SARS-CoV-2 sampling in CSF or brain tissue. However, SARS-CoV-2 has not been isolated from CSF or brain samples to date.3 In our patient cohort, only 3 patients had been tested for SARS-CoV-2 in the CSF; all findings were negative.
The most commonly reported neuroimaging finding in children with COVID-19 and MIS-C was reversible splenial lesion syndrome.4⇓⇓-7 Acute disseminated encephalomyelitis, bilateral thalamic cytotoxic lesions, and unilateral focal vasculopathy with acute infarction were other reported neuroimaging findings in pediatric patients with COVID-19.8⇓⇓-11 We did not see any of these neuroimaging findings in our patient cohort. However, patient 7 with sickle cell disease had imaging findings consistent with posterior reversible encephalopathy syndrome (which was partially resolved on subsequent brain MRI, Fig 1). This patient manifested primarily with respiratory symptoms that required mechanical ventilation. Patient 19, who had no previous history, presented with a status epilepticus and showed a right hippocampus T2-hyperintense signal (Fig 2), possibly secondary to seizure activity. This patient stayed in the intensive care unit for a MIS-C diagnosis. Attributing the positive neuroimaging findings primarily to the positive SARS-CoV-2 findings in these 2 patients with complex medical histories would be highly speculative. We believe that a 10% positivity rate of acute neuroimaging findings in our patient group implies a low yield from acute neuroimaging.
A major strength of our preliminary study is the large number of children positive for SARS-CoV-2 (n = 3694) who presented to our hospital. Limitations of this study include the following: 1) due to the retrospective nature of the study, a discrepancy between the number of neuroimaging studies and children because not each child needed follow-up neuroimaging, 2) single-center evaluation of patients, 3) still emerging data about the SARS-CoV-2 virus and its effects and still developing understanding of its consequences, 4) a positive test for SARS-CoV-2 in a patient with a neurologic symptom not necessarily meaning that the virus caused the symptom, and 5) acute neurologic symptoms being a possible selection bias.
CONCLUSIONS
Our results suggest that neurologic involvement of COVID-19 is rare among children. Only 10% of patients with neurologic manifestations demonstrated acute findings on their initial neuroimaging studies. In addition, a link between the observed imaging findings (posterior reversible encephalopathy syndrome and hippocampal edema) must still be confirmed. Of the 5 children with diagnosed MIS-C, only 1 child had an acute imaging finding (hippocampal edema). In summary, neuroimaging in children may have a low yield in COVID-19 diagnosis; consequently, requests for acute imaging should involve a careful risk-benefit analysis.
ACKNOWLEDGMENTS
The Texas Children’s Hospital COVID-19 Imaging Taskforce (Ananth V. Annapragada, Nilesh K. Desai, R. Paul Guillerman, Thierry A.G.M. Huisman, Prakash M. Masand, Gunes Orman, Amir H. Pezekhmehr, Marla B. Sammer, and Victor J. Seghers).
Footnotes
Disclosures: Ananth Annapragada—UNRELATED: Board Membership: Alzeca Biosciences; Consultancy: Alzeca Biosciences; Employment: Texas Children’s Hospital; Grants/Grants Pending: National Institutes of Health, Alzeca Biosciences*; Patents (Planned, Pending or Issued): numerous United States and foreign patent applications; Royalties: University of Texas; Stock/Stock Options: Sensulin, Alzeca Biosciences; Other: Texas Children’s Hospital also receives royalties from my inventions.* *Money paid to the institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received November 16, 2020.
- Accepted after revision December 14, 2020.
- © 2021 by American Journal of Neuroradiology