Abstract
SUMMARY: Recently, a distinct clinicoradiologic entity involving cerebellar, hippocampal, and basal nuclei transient edema with restricted diffusion (CHANTER) on MR imaging was identified. Patients present in an unresponsive state following exposure to drugs of abuse. Very little information exists regarding this entity, particularly in the radiology literature. We identify and describe 3 patients at our institution with similar clinical and radiographic findings. Multifocal restricted diffusion in the brain is typically associated with poor outcomes. By contrast, CHANTER involves intraventricular obstructive hydrocephalus that, when treated, can lead to substantial recovery. This novel syndrome should be on the differential in patients who present in an unresponsive state after recent opioid use in the context of the above imaging findings. Additional diagnoses on the differential can include ischemic stroke, hypoxic-ischemic encephalopathy, “chasing the dragon,” leukoencephalopathy, opioid-associated amnestic syndrome, and pediatric opioid-use-associated neurotoxicity with cerebellar edema.
ABBREVIATIONS:
- CHANTER
- cerebellar, hippocampal, and basal nuclei transient edema with restricted diffusion
- EVD
- external ventricular drain
- HIE
- hypoxic-ischemic encephalopathy
- OAA
- opioid-associated amnestic syndrome
- POUNCE
- pediatric opioid use-associated neurotoxicity with cerebellar edema
Cerebellar, hippocampal, and basal nuclei transient edema with restricted diffusion (CHANTER) syndrome is a recently described novel disease entity involving distinct radiologic and clinical findings. The initial case series that identified this constellation consisted of 6 patients who presented with stupor or coma in the setting of intoxication with drugs of abuse.1 The initial CT in these patients showed a consistent pattern of progressively worsening acute cerebellar edema, which eventually progressed to intraventricular obstructive hydrocephalus. MR imaging showed diffusion restriction in the bilateral hippocampi, cerebellar cortices, and basal ganglia, without substantial cortical involvement.
This combination of distinct radiologic findings and clinical course is distinct from that of other similar etiologies, such as acute ischemic stroke, hypoxic-ischemic encephalopathy (HIE), and heroin-associated spongiform leukoencephalopathy. Despite its suggestive and unique imaging features, there is a scarcity of data regarding CHANTER syndrome in the radiology literature, and clinical services often drive its diagnosis. However, prompt recognition or suggestion of CHANTER by the radiologist on MR imaging is essential. It may allow aggressive early treatment, which might not be offered in cases of HIE or multifocal diffusion restriction with obstructive hydrocephalus, given a presumed poor neurologic outcome.1,2
Preliminary data suggest an association between the use of drugs of abuse, particularly opioids, and the eventual diagnosis of CHANTER syndrome. Fentanyl overdoses are now the leading cause of death among those 18–45 years of age in the United States.3 The coronavirus disease 2019 (COVID-19) pandemic and response have caused a spike in opioid overdoses, predicted to worsen further.4 The increasing scope of the opioid epidemic elucidates the necessity for radiologists and clinicians to recognize the findings of CHANTER because the disease prevalence is expected to increase.
Since the initial series of patients with CHANTER syndrome, only 1 case report in the literature of suspected CHANTER syndrome has been published.5 Additionally, further classification has occurred for 2 syndromes that bear a resemblance to CHANTER: pediatric opioid use-associated neurotoxicity with cerebellar edema (POUNCE)6 and opioid-associated amnestic syndrome (OAA).7 Thus, the relationship of these patterns to CHANTER has yet to be discussed. We believe that our case series supports CHANTER as an essential entity that radiology can suggest on the basis of imaging.
Case Series
Patient 1.
A 54-year-old woman with a history of HIV and IV drug use presented in an unresponsive state after a fentanyl overdose. Naloxone was administered, but the patient remained encephalopathic and was intubated for airway protection.
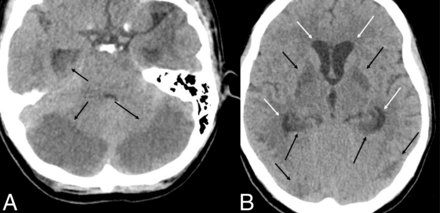
The initial CT showed subtle edema and hypoattenuation in the cerebellar hemispheres, with a developing mass effect on the fourth ventricle. There was no hydrocephalus at the time nor any hypoattenuation within the basal ganglia. The patient continued to deteriorate clinically. CT performed the day after admission showed progressive hypoattenuation and edema within the cerebellar hemispheres; new edema within the posterior hippocampi, basal ganglia, and cortex; and developing hydrocephalus (Fig 1). The patient was given IV mannitol, admitted to the neurocritical care unit, and had an external ventricular drain (EVD) placed for CSF flow diversion. CTA showed no large-vessel occlusions, and CTP data showed no evidence of core or penumbra to suggest a stroke or hypoperfusion. Continued deterioration led to the patient requiring a suboccipital decompressive craniectomy.
Representative findings on initial head CT. Axial NCCT demonstrates hypoattenuation within the cerebellar hemispheres and hypoattenuation within the posterior hippocampi (black arrows, A) and hypoattenuation within the basal ganglia, hippocampi, and cortex (black arrows, B). Developing hydrocephalus is present (white arrows, B).
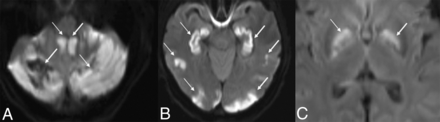
MR imaging showed areas of diffusion restriction in the bilateral cerebellar hemispheres, bilateral hippocampi, bilateral basal ganglia, and deep WM, and scattered cortical involvement (Fig 2). Blood products were seen in the right cerebellum. TOF-MRA confirmed the lack of large-vessel occlusion.
MR DWI from patient 1, thirty hours after presentation, shows diffusion restriction in the cerebellum (white arrows, A), hippocampi and cortex (white arrows, B), and basal ganglia (white arrows, C). The ADC map is not shown.
In 1 week, the patient was extubated, and her EVD was weaned and removed. The patient’s mental status was slowly improving. A repeat MR imaging 2 weeks postadmission (Online Supplemental Data) showed the evolution of known injury involving the cerebellum, temporo-occipital lobes, hippocampi, and basal ganglia with decreased T2/FLAIR hyperintensity and increased areas of enhancement, as well as increased abnormal T2/FLAIR signal within the subcortical WM of the pre- and postcentral gyri bilaterally, without enhancement, restricted diffusion, or susceptibility artifacts.
Although the patient required tracheostomy and gastrostomy, she improved enough for transfer to the floor and subsequently to a long-term acute care facility. At follow-up 5 weeks after admission, the patient had a substantially improved mental status with mild memory, speech, and gait deficits. The estimated mRS score was a 3. CT showed encephalomalacia in the bilateral cerebellar hemispheres, occipital lobes, and left parietal lobe and scattered hypodensities in the deep WM.
Patient 2.
A 33-year-old man with a medical history of treated HIV and IV drug use presented after being found unresponsive. A urine drug screen was positive for amphetamines, cocaine, fentanyl, and benzodiazepines. He was given naloxone with minimal arousal. He was subsequently intubated for airway protection, given IV hypertonic saline, and admitted to the neurocritical care unit.
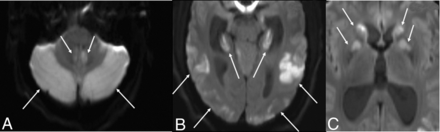
The initial CT demonstrated bilateral cerebellar hypoattenuation and hypoattenuation involving the basal ganglia, posterior hippocampi, and parieto-occipital cortex. It also showed developing obstructive hydrocephalus with partial effacement of the fourth ventricle and cerebral aqueduct as well as dilation of the lateral and third ventricles. MR imaging showed diffusion restriction in the cerebellar hemispheres, hippocampi, temporo-occipital cortex, and basal ganglia (Fig 3). TOF-MRA showed no evidence of large-vessel occlusion.
MR DWI from patient 2, six hours after presentation, shows diffusion restriction in the cerebellar hemispheres (white arrows, A), hippocampi and temporo-occipital cortex (white arrows, B), and basal ganglia (white arrows, C). The ADC map is not shown.
The patient had a posterior fossa craniectomy with resection of the cerebellar tissue and EVD placement for intraventricular obstructive hydrocephalus. His EVD was weaned and removed. An MR imaging performed 2 weeks postadmission demonstrated the expected evolution with normalization of the abnormal findings on DWI involving the cerebellum, hippocampi, and basal ganglia (Online Supplemental Data).
The patient could not be extubated secondary to mental status and ultimately had a tracheostomy. The patient improved enough for transfer to the floor and subsequently to a long-term acute care facility. CT at discharge showed resolution of hypoattenuation and mass effect in the posterior fossa. At follow-up 14 weeks after admission, the patient could follow simple commands and communicate with eye contact, and the estimated mRS score was 5.
Patient 3.
A 42-year-old woman with a medical history of polysubstance abuse presented in an unresponsive state after being found down after a fentanyl overdose. Her urine drug screen was positive for fentanyl, amphetamines, and cannabinoids. She was given naloxone and had minimal responsiveness. She was intubated for airway protection due to a somnolent mental status.
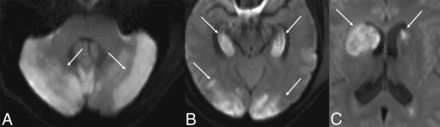
The initial CT demonstrated areas of decreased attenuation and edema involving the bilateral cerebellar hemispheres, the right basal ganglia, and hippocampi. The patient continued to deteriorate. A repeat CT the following day revealed increased prominence of the hypoattenuation and edema in the previously mentioned areas, dilation of the lateral ventricles consistent with developing hydrocephalus, and a new area of marked hypoattenuation in the left caudate. Initial MR imaging showed diffusion restriction in the bilateral cerebellum, bilateral hippocampi, cortex, and basal ganglia with blood products in the right cerebellum (Fig 4). TOF-MRA showed no large-vessel occlusions.
DWI of patient 3, fifty-eight hours after presentation, shows diffusion restriction in the cerebellum (white arrows, A), hippocampi and cortex (white arrows, B), and basal ganglia (white arrows, C). There is mass effect from the diffusion restriction in the right caudate nucleus on the frontal horn of the right lateral ventricle (C). The ADC map is not shown.
The patient had EVD placement for obstructive hydrocephalus. Her EVD was weaned and eventually removed. An MR imaging at 2 weeks postadmission showed resolving areas of the initially noted diffusion restriction, along with FLAIR hyperintensity and postcontrast enhancement within the cerebellum, hippocampi, cortex, and basal ganglia consistent with evolving injury (Online Supplemental Data).
The patient was extubated successfully but had a gastrostomy tube placed due to dysphagia. The patient’s condition improved enough for transfer to a skilled nursing facility. At discharge, the patient regarded the examiner briefly but was mute and did not reliably track or follow commands. The estimated mRS score was 5.
DISCUSSION
All 3 patients in our series had presentations, imaging findings, clinical courses, and outcomes similar to those in patients first identified and categorized as having CHANTER by Jasne et al.1 No other documented syndrome fit these findings as well as CHANTER. Thus, we concur with the classification of this unique constellation of symptoms as a distinct clinicoradiographic syndrome of CHANTER.
Radiographic Features
The 3 patients in our series had consistent NCCT findings. The initial CT findings showed cerebellar edema with eventual mass effect on the fourth ventricle and intraventricular obstructive hydrocephalus requiring shunting and decompression. A varying degree of involvement of the basal ganglia and cortex was noted on the initial CT, with subsequent development of these findings on CT the following day. All patients had vessel imaging showing no large-vessel occlusion to suggest arterially mediated infarctions. No evidence of hemorrhage was seen on NCCT in any of these cases. CTP and regional CBF images in 1 patient showed no evidence of a stroke or decreased blood flow to the basal ganglia and cerebellum. This finding suggests that the injury in CHANTER is not related to vascular flow restriction but rather likely internal cellular injury.
While patients who present in an unconscious state are likely to have NCCT as their first imaging study, MR imaging findings, especially diffusion restriction, are the distinct radiologic hallmark of CHANTER and necessary for diagnosis. The initial MR imaging findings among our 3 patients were also consistent. On initial MR imaging, all patients showed diffusion restriction in the cerebellum, hippocampi, basal ganglia, and cortex. Only 1 of the patients had subcortical WM involvement of the centrum semiovale. Initial MR imaging was performed within several hours for 2 patients but was delayed in patient 3. A follow-up MR imaging generally obtained 2–3 weeks later in all cases showed expected injury evolution with normalization of signal on DWI, FLAIR hyperintensity, and postcontrast enhancement in these areas, consistent with evolving injury. All patients had TOF-MRA demonstrating the absence of occlusion in large vessels.
The radiologic findings among the 3 patients in our series are largely consistent with those of the 6 patients in the Jasne et al1 series, including the initial CT findings, development of obstructive hydrocephalus following cerebellar edema, and diffusion restriction in the cerebellar cortex, hippocampi, and basal ganglia, typical of CHANTER. The only substantial discrepancy was the presence or absence of cerebral cortical involvement. Jasne et al reported that all 6 of their patients had the typical diffusion restriction findings but sparing of the cerebral cortex. However, all 3 patients in our series had some extent of cerebral cortical involvement, though this was not a dominant feature. The involvement of subcortical WM was rare in both case series. Only 1 patient in the series of Jasne et al showed areas of restricted diffusion in the subcortical WM. In contrast, no patients in this series exhibited such findings.
Clinical Course.
Clinicians often approach a pattern of large symmetric areas of restricted diffusion in the brain as representing severe, irreversible injury and a poor neurologic outcome with a small chance of improvement. This belief could stem from the poor prognosis in patients with multifocal diffusion restriction caused by HIE, with only one-quarter of patients with HIE surviving to hospital discharge and those surviving having severe neurologic deficits.2 While the initial presentation of patients with CHANTER syndrome is severe, the outcomes in these patients illustrate the potential for substantial clinical improvement with prompt detection and treatment of the edema.
Regarding the 6 patients classified as having CHANTER by Jasne et al,1 4 patients had EVD placement for CSF flow diversion and 3 had suboccipital decompression. Although 1 patient died during hospitalization due to brain herniation, 5 had notable levels of functional improvement. The patients in our series are all different individuals from those reported by Jasne et al.
After medical and surgical intervention, our patients had similar outcomes compared with those in the initial CHANTER series. All 3 had EVD placement, and 2 had further neurosurgical decompression. Obstructive hydrocephalus resolved in all patients. All survived and showed substantial-but-variable neurologic improvement compared with their presenting status. Similarly, Kobayashi et al,5 in 2021, described a case of CHANTER syndrome in which the patient had a dramatic clinical improvement with the only deficit being mild leg weakness. In contrast, the presence of large areas of symmetric restricted diffusion following anoxic brain injury typically predicts very poor neurologic outcome.
Differential Diagnoses and Classification
Acute ischemic stroke is unlikely to be confused with CHANTER because patients with CHANTER do not have any vessel occlusion on imaging. Additionally, patients with CHANTER typically have injuries in multiple vascular territories. A related pathology that is closer with respect to radiologic features to CHANTER is HIE. Patients with HIE can present in an unresponsive state, similar to that in patients with CHANTER. However, they do not necessarily have acute exposure to drugs of abuse. The outcomes are typically extremely poor compared with patients with CHANTER syndrome.2 This pattern of injury tends to affect areas of high metabolic demand. Notably, cerebellar and hippocampal edema can be seen in HIE. However, when this occurs, other areas, such as the cerebral cortex, are often diffusely involved.8,9 In HIE, NCCT will typically show a diffuse loss of gray-white matter differentiation and effacement of the sulci with cytotoxic edema.10,11 These findings were not seen in any patients classified as having CHANTER.
Additionally, in HIE, the cerebellum is more likely to be spared, giving rise to the white cerebellum sign, contrary to the cerebellar hypoattenuation seen on CT in all patients with CHANTER syndrome. Last, while obstructive hydrocephalus developed in all patients with CHANTER syndrome, it is not common in patients with HIE.12 However, it is possible that some patients described here and previously with CHANTER syndrome might also have some degree of superimposed HIE, which might partially account for the variability in outcomes.
Heroin-associated spongiform leukoencephalopathy is a toxic leukoencephalopathy with distinct imaging features. Because it is exclusively associated with heroin vapor inhalation, it is also known as “chasing the dragon” leukoencephalopathy. Although it can present with cerebellar restricted diffusion, it almost always presents with changes in the WM, including symmetric confluent deep WM hyperintensity on T2 and FLAIR sequences, with sparing of the gray matter, which is not typical of CHANTER.11,13 Contrary to CHANTER, it also presents during a more extended period.
Another recent case remarkably similar to CHANTER consisted of cerebellar edema in a young child following accidental opioid ingestion and fentanyl administration.14,15 It appears that this case, as well as several others in the pediatric population, may fit under the recently described constellation of POUNCE.6,16⇓⇓⇓⇓-21 Children with POUNCE are generally accidentally exposed to opioids and present with a decreased level of consciousness. Similar to CHANTER, the prominent feature of POUNCE is malignant cerebellar edema, with diffusion restriction occasionally reported.14,18,22 This cerebellar edema can lead to obstructive hydrocephalus.14 However, contrary to CHANTER, these cases rarely have hippocampal and basal ganglia involvement, instead involving WM areas with lesions that are hyperintense on T2 and hypoattenuated on CT.14,15,18,20,22 Some of these differences in injury patterns have been speculated to be due to the differences in opioid receptor expression between childhood and adulthood.23,24
Another syndrome that has some overlapping features with CHANTER is OAA. Patients with OAA present with new-onset amnesia after exposure to opioids, most notably fentanyl.7,25⇓⇓⇓-29 Radiologically, the prominent finding is symmetric diffusion restriction and FLAIR hyperintensity of the hippocampi. Edema in the cerebellum and basal ganglia occurs only rarely, and patients do not present with obstructive hydrocephalus. Presentation and outcomes are milder than in CHANTER, with amnesia as the defining deficit.
The similarities between patients with CHANTER, POUNCE, and OAA perhaps indicate that these syndromes exist along a spectrum with several overlapping clinical and imaging features and a common underlying etiology (Table). It has been thought that CHANTER represents the more severe end of this spectrum, while OAA represents a milder form.1
Proposed spectrum of opioid syndromes
Febrile infections, including acute cerebellitis, would also be on the differential. However, apart from a different clinical history, cerebellitis is more likely to have leptomeningeal and cortical enhancement in the acute setting and less likely to have extensive supratentorial involvement, for example, in the basal nuclei, cortex, and hippocampi as in CHANTER.29,30
Etiology and Mechanism
There is a clear association of the presentation of CHANTER syndrome and acute intoxication with drugs of abuse, particularly opioids. Four of the 6 patients identified by Jasne et al1 had acute exposure to opiates. In our series, all 3 patients had a fentanyl overdose. Thus, although the pathogenesis of CHANTER is still uncertain, it appears that direct opioid toxicity plays a key role. The injury pattern is thought to be due to a combination of neurotoxicity and hypoxic effects, resulting in mitochondrial failure with anoxic injury.1 Other proposed mechanisms for the effect of opioids on the cerebellum have been proposed in POUNCE syndrome, including direct oligodendrocyte toxicity, demyelination, and apoptotic upregulation.6,31,32 An underlying susceptibility to CHANTER syndrome due to genetic or environmental factors might also be present. The expression of the OPRM1 gene, which encodes the μ-opioid receptor, which is the primary target of morphine-derivative opioids, is higher in key regions affected in CHANTER, particularly the cerebellum.33⇓-35
In the adult literature, heroin-associated spongiform leukoencephalopathy has preferential cerebellar involvement. It has been postulated that this is due to the high concentration of opioid receptors in the cerebellum,22,36 which are also highly expressed in affected regions of the WM.33⇓-35 The injury in OAA is thought to be due to fentanyl-induced hippocampal excitotoxicity in the context of hypoventilatory hypoxemia.37 Accordingly, we propose that the regional topography of CHANTER might be due to an overlap of brain regions most vulnerable to both opioid toxicity and hypoxic-ischemic injury, with similar conditions falling on either side of this spectrum. However, although the patients in our case series all had acute exposure to fentanyl specifically, the exposures reported by Jasne et al1 appear more heterogeneous, including 2 not having any acute opioid exposures.
CONCLUSIONS
Because of the distinct imaging features of CHANTER syndrome, radiologists can often be the first to consider this syndrome as a possible diagnosis. We believe that CHANTER syndrome is under-recognized, to the detriment of these patients who can often experience strong neurologic improvement from baseline if identified early and provided with an urgent medical and surgical intervention. While the opioid epidemic continues to worsen and synthetic opioids like fentanyl cause an even larger portion of overdoses, this syndrome will likely only increase in prevalence. Further research can better clarify the inciting factors for CHANTER, determine a more consistent radiologic presentation, elucidate its underlying mechanisms, and implicate the possibility of CHANTER being part of a spectrum of overlapping injury patterns.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received February 26, 2022.
- Accepted after revision May 13, 2022.
- © 2022 by American Journal of Neuroradiology