Abstract
SUMMARY: Life-threatening physical abuse of infants and toddlers is frequently correlated with head injuries. A common variant of the abusive head trauma is the shaken baby syndrome. The present review article sheds light on subdural collections in children with abusive head trauma and aims at providing a recent knowledge base for various medical disciplines involved in diagnostic procedures and legal proceedings. To this end, the different subdural collection entities are presented and illustrated. The pathophysiologic background is explained. Differential and age-diagnostic aspects are discussed and summarized by tabular and graphic overviews. Two problematic constellations frequently occurring during initial CT investigations are evaluated: A mixed-density subdural collection does not prove repeated trauma, and hypodense subdural collections are not synonymous with chronicity. The neuroradiologic analysis and assessment of subdural collections may decisively contribute to answering differential diagnostic and forensic questions. In addition to more reference data, a harmonization of terminology and methodology is urgently needed, especially with respect to age-diagnostic aspects.
ABBREVIATIONS:
- AHT
- abusive head trauma
- BV
- bridging vein
- cSDH
- chronic subdural hematoma
- SDC
- subdural collection
- SDE
- subdural effusion
- SDH
- subdural hematoma
- SDEm
- subdural empyema
- SDHy
- subdural hygroma
- SDHHy
- subdural hematohygroma
In light of serious physical, psychological, and legal consequences, physical child abuse attracts increasing attention in terms of health policy and health economy.1⇓–3 Head injuries represent the most frequent cause of lethal outcome and mainly relate to children within their first and second years of life.4⇓–6 Currently, the term “abusive head trauma” (AHT) is used for any nonaccidental or inflicted head injuries in pediatrics.7⇓–9
AHT has a worldwide incidence of 14–30/100,000 live births among children younger than 1 year of age.5,10⇓⇓–13 Additionally, a high amount of underreporting has to be assumed because many cases are not identified due to subclinical courses, nonspecific symptoms, or missing medical consultation.14 Meta-analyses on the outcome revealed an average mortality rate of around 20% among children younger than 2 years of age.15 Survivors showed severe disability (eg, tetraplegia, epilepsy, or blindness) in ∼34%, and moderate disability (eg, hemiplegia, memory and attention difficulties) in ∼25% of the cases.15
The shaken baby syndrome—a common variant of AHT with increasing general public awareness—is characterized by the following features that are neither obligatory nor evidentiary:
Acute encephalopathy, being the clinical expression of traumatic damage of the brain parenchyma accompanied by a wide spectrum of neurologic symptoms that depend on the intensity of the trauma.
Subdural collections with or without additional extra-axial findings such as subarachnoid hemorrhage, arachnoid tear, or bridging vein thrombosis.
Retinal hemorrhages typically found in many locations, within several layers, disseminated, widespread from the center to the periphery, and with or without additional retinoschisis or intravitreal hemorrhage.
Spinal trauma such as ligamentous injuries at the craniocervical junction, or spinal sub- or epidural hematomas.
No or only minimal injuries of the skin because skin bruises caused by firm grip at the arms or the thorax of the child are rare.
Missing or inadequate anamnesis—that is, no trauma reported or report of just a minor trauma despite the presence of severe brain injury.
With respect to other variants of AHT, further features of head injury may occur, in particular, signs of blunt force (impact) trauma against the child's head such as skin lesions or skull fractures.
Relevant differential diagnoses such as metabolic disorders, infectious and hematologic diseases, and birth trauma must be excluded. However, these differential diagnoses usually cannot explain the symptomatology of AHT as a whole. Diagnosing AHT always requires the joint assessment of numerous investigation results from pediatrics, ophthalmology, neurosurgery, laboratory medicine, forensic medicine, and radiology.7,16 Pediatric neuroimaging by CT and MR imaging plays a key role in this strategy.7,16,17 Traumatic brain injuries and extra-axial indicators of AHT can be depicted and evaluated across time. Besides subarachnoid hemorrhages, fluid collections within the subdural space represent such extra-axial indicators of AHT.
Subdural Collections
The term “subdural collection” (SDC) is understood as a nonspecific umbrella term comprising various, in part, successively stagelike findings within the subdural space. The radiologic investigation of SDCs has the potential to contribute to important issues such as type, number, and circumstances of the traumatic force or the age of injury. Apart from the clinical and medicolegal significance for the diagnosis of child abuse, SDCs may also be relevant for criminological aspects because age estimation possibly facilitates further limitation of the circle of suspects.
Differential diagnostics of the various SDC entities is a challenging topic for the radiologist. During the initial image-assessment process, the more careful labeling as SDC may be more reasonable than the possibly hasty determination of a special SDC entity.18,19 Terminology and definition criteria of the SDC entities are inconsistent, even among experts. This issue may partly be attributed to the frequent presence of mixed or transitional SDC forms. However, the large body of literature allows the differentiation of at least the following 6 entities.
Subdural Hematoma
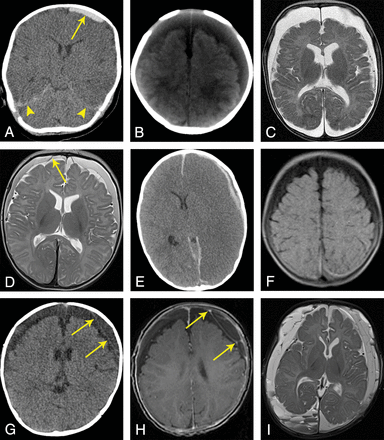
In the context of AHT, subdural hematoma (SDH) is described as the most common intracranial pathology in infants and toddlers.20⇓–22 SDHs, like all SDCs, may occur unilaterally or bilaterally.23 The convexities of the cerebral hemispheres (Fig 1A), the falx cerebri, the tentorium cerebelli, and the middle and posterior cranial fossae are considered typical locations.22 In many cases, SDHs have a key role as a diagnostic marker only—that is, though they may represent an important symptom of child abuse, their volumes are often small, resulting in just a minor space-occupying effect.8,21,23⇓–25 Hence, frequently, SDHs do not have a prognostic relevance for the extent of brain damage.24 Depending on the developmental stage in which subdural blood is visualized by neuroimaging, SDHs have a wide variety of appearances (Table 1). The chronic SDH has a special position (see below: “Chronic Subdural Hematoma”).
SDC entities in AHT cases. A, Acute SDH (nonenhanced CT): a 2-month-old boy with a small hyperdense SDC over the left frontoparietal region (arrow) and hyperdense blood components around the tentorium (arrowheads). B, SDHy (nonenhanced CT): a 2-month-old boy with wide, homogeneously hypodense (or CSF-isodense) SDCs over both frontoparietal regions; no neomembranes or septa. C, SDHy (MR imaging, T2WI, TSE, nonenhanced): a 4-month-old boy with wide, homogeneously CSF-isointense SDCs over both frontoparietooccipital regions, markedly frontal due to the supine position; no neomembranes or septa. D, SDHHy, homogeneous variant (MR imaging, T2WI, TSE, nonenhanced): a 3-month-old boy with homogeneous SDCs over both frontoparietal regions. Compared with CSF within the external and internal CSF spaces, the SDCs appear hypointense. Two intact BVs can be found next to the superior sagittal sinus (arrow shows 1 BV). E, SDHHy, heterogeneous variant (nonenhanced CT): a 19-month-old boy with an SDC in the left frontoparietal region. The SDC is composed of a thin, brain-sided, hyperdense component and a thin, dura-sided, hypodense component that runs parallel to the former component (mixed-density pattern). In this case, the study also revealed severe brain edema with a midline shift to the right side as well as hyperdense blood components within the anterior and posterior interhemispheric fissures. F, SDHHy, heterogeneous variant (MR imaging, FLAIR, nonenhanced): a 4-month-old girl with wide SDCs over both frontoparietooccipital regions. While the frontoparietal SDC proportions appear hypointense, the parietooccipital proportions are iso- to hyperintense. The transitional zone between the 2 components is almost smooth; fluid-fluid levels cannot be recognized unambiguously. G, Chronic SDH (nonenhanced CT): a 7-month-old boy with wide, hypodense SDCs over both frontoparietal regions and subtle formation of subdural neomembranes on the left side (arrows). H, Chronic SDH (MR imaging, T1WI, gradient-echo sequence, enhanced by contrast agent): same case as in G. Confirmation of the presence of subdurally located septa and chamber formations. In contrast to the nonenhanced T1WI (not shown), this contrast agent–enhanced study revealed focal signal enrichment located at the neomembranes (arrows). I, Chronic SDH (MR imaging, T2WI, TSE, nonenhanced): a 4-month-old boy with numerous subdural septa and neomembranes. Note the different signal intensities and multiple fluid-fluid levels within subdural chamber formations, especially in the right occipital region.
Classic SDH stages in CT and MRI (at 1.5T)a
Subdural Hygroma
The term subdural hygroma (SDHy) is classically reserved for proteinaceous, clear, pink-tinged, or xanthochromatic collections within the subdural space containing pure CSF or at least CSF-like fluid; blood, blood products, or neomembranes are nonexistent by definition (Fig 1B, -C).22,26,27 However, the smallest amounts of blood within the SDHy cannot always be excluded and may become noticeable on CT by a slightly higher density compared with CSF (see below: “Subdural Hematohygroma,” “homogeneous variant”).
Subdural Hematohygroma
Subdural hematohygromas (SDHHys) are a combination of blood (or blood products) and CSF (or CSF-like fluid).22,28⇓–30 A homogeneous and a heterogeneous variant can be differentiated.
In many cases of an SDC diagnosed as SDHy, it may be assumed that the SDC is actually the homogeneous variant of the SDHHy (Fig 1D) because the blood component may sometimes be relatively small and/or very “young” (hyperacute); furthermore, an intense mixture of blood and CSF may be present.27⇓–29 Hence, in our experience, SDHy and SDHHy are used interchangeably or synonymously in radiology reports.
The heterogeneous variant of the SDHHy (Fig 1E, -F) indicates 2 SDC components that coexist within the same subdural compartment (eg, above a brain convexity); these components may be clearly distinguished from one another (fluid-fluid levels possible) and may appear hyper- and hypodense during CT investigations (mixed-density pattern).22,28,30⇓–32 The hypodense component is interpretable as the following:
Acute CSF collection (eg, due to an arachnoid tear, see below: “Pathophysiology”)
Supernatant (and thus an integral part of blood) changed by gravity (serum separation/blood sedimentation/hematocrit effect), in the sense of an SDH.
Of course, a mixed form of both variants is conceivable as well (ie, simultaneous presence of CSF influx and blood sedimentation; see below: “Mixed-Density SDCs: Repeated Trauma?” and Table 2, upper part).
Mixed-density and hypodense SDCs—2 typical problem constellations during the initial CT investigationa
Chronic Subdural Hematoma
Currently, from the pathophysiologic point of view, chronic subdural hematoma (cSDH) is considered a separate SDC entity.23 cSDH denotes a serosanguinous, petroleum-, or crankcase-like fluid collection surrounded and sometimes loculated (divided into compartments) by neomembranes (Fig 1G, -I).26,33⇓–35 Neomembranes contain numerous new blood vessels leading to accumulation of contrast agent in neuroimaging studies.22,27 The presence of neomembranes represents an important criterion for distinguishing cSDH and SDHy. In contrast to the situation in adults, genuine cSDHs are relatively rare in infants.22,36⇓–38
Subdural Effusion and Subdural Empyema
These proteinaceous SDC entities are predominantly considered sequelae (in case of subdural effusion [SDE]) or complications (in case of subdural empyema [SDEm], eg, due to an infected SDE) of bacterial meningitis or sinusitis.22,39 These conditions usually do not cause diagnostic difficulties because inflammatory symptomatology or a history of CNS infection is typically present. Normally, SDEs and SDEms are nontraumatic, but in rare cases, SDEms may originate following penetrating head trauma or craniotomy, which, of course, is usually known in the clinical setting.
Pathophysiology
AHT is predominantly caused by acceleration-deceleration trauma, blunt force trauma (impact), or a combination of these mechanisms.8,9,40 In acceleration-deceleration trauma, the child is usually held firmly at the thorax or upper arms and is then shaken. These rapid movements result in repeated acceleration and deceleration of the child's head due to missing postural control. Shearing and rotational forces may cause severe injuries within the brain tissue, determining prognosis. In addition, small and medium-sized blood vessels within the cranial cavity, particularly the bridging veins (BVs) that mainly run through the subarachnoid space, may rupture in part or completely. Approximately 50 BVs (diameter, 0.05–3.07 mm) connect the cortical veins of the cerebral and cerebellar surface with the large venous sinuses, thereby penetrating the inner part of the dura mater.41 Typically, injuries of the BVs cause extra-axial hemorrhage, predominantly within the subarachnoid and subdural spaces.23,41⇓⇓–44
BVs show a different wall thickness at different locations. While the BV wall measures 50–200 μm within the subarachnoid space, the BV segments that penetrate the dura mater may have a wall thickness of only 10 μm and do not show additional external strengthening by connective tissue.45 Thus, increased vulnerability of dural BV portions is assumed.45 The resulting hemorrhage from the injured BVs fosters opening of the subdural space. This pathologic space does not exist under physiologic conditions and has been recognized as an intradural lesion caused by cleavage of the innermost part of the dura mater, the dura border cell layer.22,46,47 Nevertheless, the traditional term “subdural” is still widely in use; thus, BV hemorrhage leads to what is generally referred to as SDH.
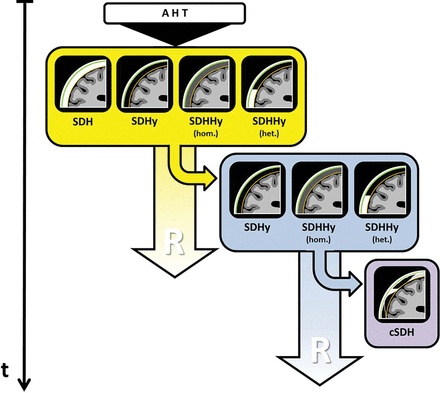
Due to shearing forces, the arachnoid membrane may also tear (eg, in the vicinity of strained BVs or at Pacchionian granulations).29,48 If this is the case, transfer of CSF from the subarachnoid space to the subdural space is possible. Thus, an SDHy or SDHHy may develop additionally or subsequently (yellow box in Fig 2).27,29,49 The laceration of the arachnoid membrane may function as a valve preventing backflow of CSF.49,50 Besides this rapid mechanism, occurring within a few minutes or hours, delayed formations of SDHys and SDHHys, requiring up to several days, have been observed as well.27 Etiopathologically, there are 2 causative mechanisms:
Influx of CSF or CSF-like fluid as a result of a posttraumatic, reactive, vasomotoric (diffusion) disorder within surrounding meningeal structures. This is assumed to occur particularly with decreased intracranial pressure and through the mediation of cytokines.20,22,33,34,51,52
Pathologic accumulation of intradural CSF that is assumed to move physiologically within the dural venous plexus from the subarachnoid space to the venous sinuses.47
Simplified schematic drawing of the development of cSDHs via SDHys/SDHHys according to Hymel et al,20 Hedlund,22 Wittschieber et al,27 Zouros et al,29Lee et al,34 and Lee.49 The findings within the yellow box demonstrate the possible SDC entities following AHT that can often be found during initial cross-sectional neuroimaging. A portion of these cases develops further toward the findings shown within the blue box. With time, these SDC entities may then develop into a cSDH (purple box). The pictographs schematically visualize the CT morphologic appearance of the respective SDC. Green indicates the dura mater; orange, the arachnoid membrane; the space in between, the subdural space; hom., homogeneous; het., heterogeneous; t, time; R, resorption/resolution.
Likewise, the further development toward cSDH is not yet completely understood. However, it seems clear that an outer subdural neomembrane (at the dural side) is primarily formed. Its formation commences at the innermost cell layer of the dura mater cleaved by the SDC. Subsequently, an inner subdural neomembrane (on the arachnoid side) is formed so that, finally, both neomembranes surround the SDC.53⇓⇓–56 Particularly the thicker outer neomembrane is highly vascularized. Leakage of these fragile new blood vessels is held responsible for additional influx of blood, proteins, and fluid and, thereby, for the increase in size of the cSDH.39,52⇓⇓⇓–56 Formation of septa is considered a consequence of repeated rebleeding events and may lead to chamber-like structures with multiple fluid-fluid levels appearing differently with regard to density or signal intensity (Fig 1I).57 A pathologically expanding SDHy or SDHHy is considered the precursor of the cSDH (blue box in Fig 2).20,22,27,34,49 The direct conversion of an acute SDH into a cSDH is infrequently observed in adult cases only and could not be simulated in animal experiments.20,58,59
Age Diagnostics
General Aspects
Given the inherent heterogeneity of traumatization and the resulting diversity of SDC appearance and SDC combinations, precise dating of SDCs based on neuroimaging alone is unrealistic. However, this issue does not mean that any time-related statements on SDCs are impossible. Hence, it seems appropriate to use more reserved terms such as “age estimation” or “staging.”60
There is general consensus that when interpreting initial imaging studies (mostly CT), SDC features should be described merely (eg, hypodense, isodense, hyperdense, or mixed-density pattern).22,28 The possibly rash labeling with temporal assignments such as “acute” or “chronic” should be avoided.22,28 In case of the sedimentation of an SDH (or SDHHy), evaluating the sediment instead of the supernatant has been recommended.31
Table 1 shows a compilation of the classic SDH stages based on relatively few data found in the literature.22,28,61⇓⇓⇓⇓–66 CT and MR imaging are regarded as complementary methods, which are both indispensable.28,32
At present, this insufficient data situation is the most limiting factor preventing more accurate age estimation by neuroimaging.37 Resilient reference data on SDH stages can rarely be obtained due to the difficult validation of the time of trauma and the highly variable severity of the injuries. Thus, the combination of insufficient reference data, little specific experience (eg, due to usually low AHT case numbers in nonuniversity institutions), heterogeneous pathophysiologic/anatomic knowledge, and general lack of consensus concerning methodology (missing guidelines) unsurprisingly results in inconsistent assessments among radiologists as shown recently.67,68 These studies reflect the poor data situation and demonstrate the broad and overlapping time intervals of SDH stages, which represent a general argument against age estimation of SDCs.67,68
However, the application of a “minimum age concept” might be an improvement towards an age-diagnostic assessment of the SDC, despite overlapping time intervals of stages. The principle is as follows: If a stage X (eg, “chronic”) is found, according to available study data, a minimum time Y (eg, 2 weeks) has elapsed since the trauma has occurred. The fact that the maximum duration of the antecedent stage often overlaps the earliest occurrence of the next stage does not affect the forensic statement (eg, that the SDC is at least 2 weeks old).
The observation of SDC development could be another possibility to increase the accuracy of age estimations of SDCs. To this end, repeated cranial imaging investigations (serial neuroimaging) are required, as long as the clinical state of the patient allows these procedures.22,31,69,70
Thus, more reliable age-diagnostic assessments of SDCs necessitate more reference studies and special training programs, imparting specific diagnostic experiences. These would also require a harmonization of methodology and terminology as a precondition. Furthermore, focusing on the density or signal intensity of SDCs alone represents only 1 approach. Other imaging findings might have the potential to support the age estimation of AHT cases in the future—that is, parenchymal shear injuries, bridging vein thromboses/venous injuries, brain edema, subdural neomembranes (see also below: “Hypodense Subdurals: Acute or Chronic?”), the size of the SDC, or other signs of brain damage.22,63,71 However, as long as large systematic studies on these topics are missing, being cautious with time-related statements on SDCs is recommended.
Mixed-Density SDCs: Repeated Trauma?
In initial CT investigations, SDCs frequently show a mixture of hyper- and hypodense proportions (so-called mixed-density pattern) (Fig 1E). This pattern is significantly more frequent in AHT than in accidental head trauma.30,72 In the past, the dogma was that such a pattern would represent a combination of “new” and “old” blood, indicating repeated trauma. Today, this view has changed. At least 4 different scenarios have been proposed as explanations for the mixed-density pattern, and 3 of them may be deduced from only 1 single traumatic event (Table 2, upper part).22,28
Scenario 4 (“acute-on-chronic” variant in Table 2) can often be excluded when an acute severe shaking event is suspected because acute rebleeding from cSDH-associated neomembranes is not associated with the typical acute symptomatology of AHT.22,24 Then, additional MR imaging and serial neuroimaging may provide more information.68,69
In the context of the mixed-density pattern, it has been proposed that SDCs with 2 different densities in “2 distant locations” may be considered indicators of a so-called “age-different pattern”60,73—that is, a hypodense frontoparietal SDC in combination with a hyperdense SDC in the posterior fossa, or a hypodense frontoparietal SDC associated with hyperdense clots at the vertex.60 Those patterns were reported to be strongly associated with confessions of repeated episodes of violence against the child, suggesting that at least 2 traumatic events occurred. However, there are numerous reports of hypodense SDCs that formed very early after the reported traumatic event (partly even within a few hours), namely without an additional trauma and also on the contralateral side of a hyperdense SDC observed initially.22,27,28,37,69,70 One possible explanation for those observations may be arachnoid tears resulting in CSF accumulations within the subdural space corresponding to acute formation of an SDHy or SDHHy.
Hypodense Subdurals: Acute or Chronic?
The presence of isolated iso- to hypodense SDCs is another typical problem in CT investigations of SDCs (Fig 1B). At least 5 possibilities of interpretation, besides SDHy and SDHHy, compose nearly all time-related SDH stages from hyperacute to chronic (Table 2, lower part).22 Hence, a reliable diagnosis and age estimation of the SDC are frequently not possible without additional MR imaging and serial neuroimaging, respectively. The diversity of differential diagnoses shown in Table 2 illustrates that the diagnosis of a chronic process (cSDH) may be hasty.
Finally, in many cases, the question is whether the diagnosis is SDHy or cSDH. While the former is compatible with both a rapid and a delayed process, the latter, in fact, suggests a traumatic event that occurred weeks ago. Several distinguishing criteria have been proposed (Table 3) to address this question.22,27⇓–29,33,66,74 The most important criterion is the presence of subdural neomembranes, septa, or chamber-like formations characterizing cSDHs. In neuropathology, the first formation of neomembranes is described as macroscopically visible after ∼10 days.74 Their radiologic detection may be challenging and often requires MR imaging, sometimes even supported by intravenous contrast. In those cases, the presence of neomembranes is described after ∼2–4 weeks.22
Possible distinguishing criteria between SDHy and cSDH in neuroimaginga
Conclusions
SDCs in infants and toddlers represent frequently occurring indicators of AHT. The radiologic analysis and assessment of SDCs remain a challenging task because different SDC entities may appear radiologically very similar at different developmental stages.
As long as no harmonization of terminology, methodology, and age diagnostic criteria of SDCs exists and as long as the scientific data situation has not improved, only rough time-related statements on SDCs will be possible. However, such statements may be helpful if a “minimum age concept” is applied. For example, it is possible to exclude that wide hypodense SDCs with neomembranes formed 2 days ago as suggested by a witness.
In summary, as consensually corroborated by a number of leading medical societies,75 the close cooperation and joint evaluation by clinicians, radiologists, and forensic experts remains essential in cases of suspected AHT.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received June 18, 2018.
- Accepted after revision August 16, 2018.
- © 2019 by American Journal of Neuroradiology