Abstract
BACKGROUND AND PURPOSE: The visual rating scales for perivascular spaces vary considerably. We sought to develop a new scale for visual assessment of perivascular spaces and to further describe their distribution and association with white matter hyperintensities in old age.
MATERIALS AND METHODS: This population-based study included 530 individuals who did not have dementia and were not institutionalized (age, ≥60 years or older; mean age, 70.7 years; 58.9% women) who were living in central Stockholm, Sweden. A semiquantitative visual rating scale was developed to score the number and size of visible perivascular spaces in 7 brain regions in each hemisphere. A modified Scheltens visual rating scale was used to assess white matter hyperintensities.
RESULTS: The global scores for perivascular spaces ranged from 4–32 for number, 3–22 for size, and 7–54 for the combination of number and size. The weighted κ statistics for the intra- and interrater reliability both were 0.77. The global score for the number of perivascular spaces increased with advancing age (P < .001). The scores for the number of perivascular spaces in the basal ganglia and subinsular regions were significantly correlated with the load of white matter hyperintensities, especially in lobar and deep white matter regions (partial correlation coefficients, >0.223; P < .01).
CONCLUSIONS: The new visual rating scale for perivascular spaces shows excellent intra- and interrater reliability. The number of perivascular spaces globally and, especially, in the basal ganglia, is correlated with the load of lobar and deep white matter hyperintensities, supporting the view that perivascular spaces are a marker for cerebral small-vessel disease.
ABBREVIATIONS:
- ICD
- International Classification of Diseases
- PVH
- periventricular hyperintensities
- PVS
- perivascular spaces
- SNAC-K
- Swedish National study on Aging and Care in Kungsholmen
- STRIVE
- STandards for Reporting Vascular changes on nEuroimaging
- WMH
- white matter hyperintensities
The perivascular spaces (PVS) are subpial virtual spaces between the adventitia of the vessel and the basal membrane of the glia limitans, involved in the lymphatic drainage of the brain.1,2 PVS are now easily identifiable in vivo, owing to improved MR imaging techniques.3 Since the late 1980s, different rating scales have been developed for the evaluation of PVS, depending on the purpose of the study.4⇓⇓⇓⇓–9 However, very few scales have simultaneously taken the number, size, and location of PVS into consideration. This is important because it remains unclear whether the number or the size of PVS is clinically relevant. Also, PVS in various anatomic regions of the brain may differ in risk factors, neuropathologies, and functional consequences.10,11 Thus, STandards for Reporting Vascular changes on nEuroimaging (STRIVE) have proposed to evaluate the number, size (maximum diameter), and anatomic location of PVS.12
PVS are common among elderly adults. Indeed, PVS are correlated with increasing age,4,9,13 though some earlier studies have shown equal distribution of PVS through a range of ages.14,15 In addition, a sex difference in the distribution of PVS in the basal ganglia has been reported, with men having more PVS than women.11,13,16 The most common etiologies proposed for the widening of the originally virtual PVS to become visible on MR imaging are hypertension13,17 and amyloid deposition, at least in patients with Alzheimer disease.18 Some studies have suggested that PVS are associated with cognitive decline and dementia.6,13
In the past decade, studying PVS as an imaging marker for cerebral small-vessel disease represents one of the research frontiers in brain aging.8 Some studies have reported that PVS are correlated with the load of both white matter hyperintensities (WMH) and periventricular hyperintensities (PVH) independent of age and vascular risk factors,19 suggesting that PVS may be an imaging marker for cerebral small-vessel disease. However, it has been unclear whether PVS in various anatomic regions are differentially associated with global and regional WMH because PVS in different regions may have different origins and etiopathologies.10
Here we present data from a population-based MR imaging study within the Swedish National study on Aging and Care in Kungsholmen (SNAC-K) in central Stockholm, Sweden.20 Within this study, we have the unique opportunity to describe a new visual rating scale for PVS by considering the number, size, and location of PVS. Furthermore, we sought to investigate the age- and sex-specific distribution of PVS in older individuals and to explore whether global and regional PVS are associated with the load of global and regional WMH.
Materials and Methods
Study Participants
Participants were derived from the SNAC-K study, a multidisciplinary study of aging and health, as fully reported elsewhere.20 Briefly, the SNAC-K study included 4 younger cohorts with 6-year intervals (60, 66, 72, and 78 years of age) and 7 older cohorts with 3-year intervals (81, 84, 87, 90, 93, 96, and 99 and older years of age). Of all 4590 persons who were eligible to participate in SNAC-K, 3363 (73.3%) were eventually examined at baseline (March 2001 to June 2004). From September 2001 to October 2003, five hundred fifty-five SNAC-K participants who were not institutionalized or disabled and who did not have dementia also underwent brain MR imaging examinations.20 Of these, 2 persons did not complete the entire examination owing to claustrophobia, 4 had suboptimal MR imaging because of motion artifacts, 2 were excluded because of large meningiomas, and images for 17 persons were lost due to technical problems. Therefore, 530 subjects were included in this study. All the participants had a Mini-Mental State Examination score of ≥21 (mean score, 29). Fifteen brain regions in 6 subjects were impossible to assess because of large infarcts. Therefore, these regions, but not other regions in these subjects, were excluded from our analysis.
SNAC-K was approved by the regional ethics review board in Stockholm, Sweden. Written informed consent was collected from all participants in the SNAC-K MR imaging sample.
MR Imaging Acquisition
All participants were scanned on an Intera 1.5T system (Philips Healthcare, Best, the Netherlands). For the visual assessment of PVS, a T1-weighted sequence (MPRAGE; TR, 15 ms; TE, 7 ms; flip angle, 15°) consisting of one hundred fifty 1.5-mm axial sequential images without angulation; a FLAIR sequence (TR, 6000 ms; TI, 1900 ms; TE, 100 ms; echo-train length, 21; flip angle 90°) of twenty 5-mm sequential images, with a gap of 1 mm, angled to the subcallosal line; and a proton-density/T2-weighted sequence (TR, 3995 ms; TE, 18/90 ms; echo-train length, 6; flip angle, 90°) of sixty 3-mm sequential images without a gap or angulation were used. All images were reviewed on a clinical PACS system.
Visual Assessment of PVS
We constructed a visual rating scale while considering the number, size, and location of PVS. The assessment was performed separately for each hemisphere, and the different locations of the brain were chosen on the basis of the regions used in earlier studies.6,7,21 In each region of both hemispheres, the number and maximum diameter of PVS were scored and recorded separately. The scores for both the number and size were then summed up to obtain a global semiquantitative measurement of visible PVS, with a maximum score of 84.
The brain regions scored were the cerebellum, mesencephalon (including the upper part of pons), hippocampus, subinsular territory (external capsule, claustrum, and extreme capsule), basal ganglia (medial to external capsule, including the thalamus and internal capsule), frontal lobe, and parieto-occipital lobe. Visible PVS in each region were counted and then scored as 0 (no visible PVS), 1 (1–5 PVS), 2 (6–10 PVS), or 3 (>10 PVS) (Fig 1 and On-line Table 1). When we measured the diameter of obliquely imaged PVS, the smallest diameter of each periventricular space was used. The maximum diameter of PVS was scored as 0 (no visible PVS), 1 (1–2 mm), 2 (3–4 mm), and 3 (>4 mm). All images were assessed by an experienced clinical neuroradiologist (A.L.). Six months after the initial reading, MR images of 20 randomly selected subjects were re-evaluated for PVS, which yielded a weighted κ statistic of 0.77 (intrarater reliability). In addition, MR images of 18 subjects were evaluated for PVS by an experienced general radiologist (L.B.); this evaluation yielded a weighted κ statistic of 0.77 (interrater reliability).
Sample images of grades 0–3 for the number of perivascular spaces bilaterally in the basal ganglia medial to the external capsule. The images indicate grade 0 (A), grade 1 (B), grade 2 (C), and grade 3 (D) in the basal ganglia. Note that the perivascular spaces contributing to the score might be in another level and thus not visible in the sample images.
We used the T1-weighted 3D volume images to count the total number of PVS in the basal ganglia and subinsular region and followed them through the sections, which facilitated separating PVS from lacunes and choroidal fissure cysts and avoided counting the same space twice. With T1, instead of T2, images for the basal ganglia also diminished the disturbance from WMH common in this region that might be confused with PVS. The PVS in the frontal and parietal lobes were more easily assessed on the axial T2 images as well as hippocampal sulcus remnants and PVS in cerebellum and mesencephalon (Fig 2). The FLAIR images were used to distinguish PVS from lacunes. The changes were considered to constitute lacunes if they were surrounded by a high signal on FLAIR images or if they did not have a rounded elongated shape. However, because the scale is based on counting the number and measuring the diameters of PVS, different sequences with thin sections might be used.
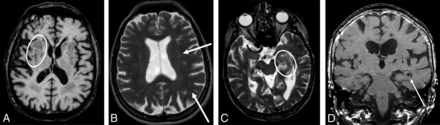
Sample images of PVS with different grades. A and B, Grade 3 in number and grade 1 in size of PVS in the subinsular, basal ganglia, frontal, and parietal regions. There are >10 PVS in each region, but none of the PVS are wider than 2 mm. In the basal ganglia, PVS are seen as dots (oval) because the image is acquired cross-sectional to the vessels (A, axial T1), while PVS are linear in the frontal and parietal lobe (arrows), where the vessels are in a plane with the image (B, axial T2). C, Grade 3 in number and grade 1 in size in the left hippocampus (oval, axial T2). PVS in the hippocampus are really hippocampal sulcus remnants, which can be clearly seen in D (arrow, coronal T1).
Visual Assessment of WMH and PVH
WMH were defined as hyperintense signal on FLAIR images. The load of WMH was rated using a modified Scheltens rating scale (On-line Table 2).22 The rating scale is the same as the original Scheltens scale, but the regionalization is different. First, we rated the 2 hemispheres separately. Second, due to very few white matter changes in the thalami and the cognitive importance of the external capsule, the basal ganglia and thalami were divided into subinsular region (external capsule, claustrum, and extreme capsule) and basal ganglia (medial to external capsule, including the thalamus and internal capsule). Hyperintense changes in the basal ganglia and thalami were included in the WMH, as previously suggested.23 Finally, we combined the parietal and occipital lobes into 1 region due to the low number of WMH in the occipital lobe. PVH were rated according to the original Scheltens scale.22
All assessments of WMH were completed by a clinical neuroradiologist (A.L.) without knowledge of subjects' clinical characteristics. The intrarater reliability for WMH was good, with the weighted κ statistic of 0.67.
Assessment of Cardiovascular Health
Data on demographics (eg, age, sex, and education) and cardiovascular risk factors and disorders were collected through face-to-face interviews, clinical examinations, laboratory tests, and patient registers.20 Hypertension was defined as arterial blood pressure of ≥140/90 mm Hg or current use of antihypertensive agents. Obesity was defined as a body mass index of ≥30 kg/m2. High total cholesterol was defined as a total serum cholesterol of ≥6.22 mmol/L or use of cholesterol-lowing agents. Diabetes was defined as having a self-reported history of diabetes, a record of diabetes in the patient register, use of hypoglycemic agents, or an HbA1c of ≥5.4%.
A history of heart disease was ascertained according to medical records in the Stockholm patient register. We included coronary heart disease (International Classification of Diseases, Ninth Revision [ICD-9] codes 410–414; ICD-10 codes I20-I25); heart failure (ICD-9 code 428 and ICD-10 code I50); and atrial fibrillation (ICD-9 code 427.8 and ICD-10 code I48).
Statistical Analysis
Demographic characteristics of study participants by sex were compared using the t test for continuous variables and the χ2 test for categoric variables. We used a scatterplot to show the distribution of global PVS scores by age, and the correlations of PVS scores with age were explored with the general linear regression model. To demonstrate the distribution of the regional PVS load, we presented the number and proportion of subjects according to the scores of PVS in each brain region for both hemispheres. Partial correlation was used to investigate the correlation between PVS scores and WMH scores globally and by regions, controlling for age, sex, and education. STATA, Version 12.0 for Windows (StataCorp, College Station, Texas) was used for all analyses.
Results
Characteristics of Study Participants
Table 1 shows the demographic and clinical characteristics of the 530 participants by sex. The mean age of the sample was 70.7 ± 9.1 years, and 58.9% were women. Men were more likely than women to have a higher level of education (P < .01), diabetes (P < .01), and coronary heart disease (P = .02). There were no significant sex differences in the mean age, prevalence of hypertension, high total cholesterol, obesity, atrial fibrillation, and heart failure or in the severity of WMH and PVH (P > .10) (Table 1).
Demographic and clinical characteristics of study participants by sex
Distribution of Global PVS
The means of the global score for the number of PVS were 18.7 ± 5.2 (range, 4–32); 10.7 ± 2.6 for the size of PVS (range, 3–22); and 29.3 ± 7.4 for combining the number and size of PVS (range, 7–54). The PVS total score shows approximately normal distribution (On-line Figure). Large PVS (>4 mm) were detected in only 6 individuals (1.1%) of all the 530 participants or in 29 regions (0.4%) of a total number of 7405 brain regions (each person having 7 regions in each hemisphere).
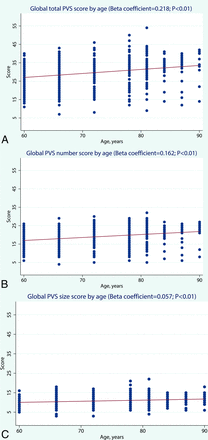
The global score for the combination of both the number and size of PVS increased with advancing age (β coefficient = 0.218, P < .01) as did the global score for the number of PVS (β coefficient = 0.162, P < .01). The global score for PVS size did not vary substantially with age, though the correlation was statistically significant (β coefficient = 0.057, P < .01) (Fig 3). There was no significant sex difference in the distribution of global scores for either the number or size of PVS.
Age-specific distribution of global scores for the number, size, and a combination of both number and size of PVS. Note that there are 3 subjects with missing values in the global PVS score for size.
Distribution Patterns of Regional PVS
Around 60% of the subjects showed >10 PVS in the frontal and parieto-occipital lobes; ∼45% showed 6–10 PVS in the basal ganglia; >50% showed 1–5 PVS in the subinsular, hippocampus, or mesencephalon regions; and >85% of the subjects did not have any PVS in the cerebellum region (Table 2).
Distribution of regional perivascular spaces by number and sizea
For distribution of regional PVS by size, >80% of the subjects had a PVS size of 1–2 mm in the frontal lobe, parieto-occipital lobe, or basal ganglia. The most common region for having PVS of ≥3 mm was the basal ganglia (∼15% in both hemispheres). The distribution of PVS by both number and size appeared to be symmetric in both hemispheres.
Correlation between WMH and PVS
After we controlled for demographics, the global scores for the number, size, and both the number and size of PVS were all positively correlated with the WMH score in lobar and deep white matter areas (P < .05 or P < .01), whereas there was no significant correlation between global PVS and PVH scores (Table 3).
Partial correlation coefficients between PVS and WMH scoresa
Higher scores for the number of PVS in the basal ganglia and subinsular areas were significantly associated with a higher global WMH score and a higher WMH score in the lobar, deep, and periventricular areas. Furthermore, a higher score for the number or size of PVS in the hippocampus was significantly associated with higher WMH scores in lobar areas. Finally, PVS scores in the frontal lobe, parieto-occipital lobe, mesencephalon, and cerebellum were largely not correlated with WMH scores.
Discussion
In this population-based study of older adults, we have developed a new rating scale for visual assessment of PVS, considering the number, size, and location, which meets the requirements of STRIVE, the neuroimaging standards for research into cerebral small-vessel disease.12 With this scale, we show evidence that PVS are an imaging marker for cerebral small-vessel disease and that the number, rather than the size, might be a more important measure for PVS. In addition, using this scale, we will be able to investigate different risk factors for and functional consequences of PVS by various brain areas.
When we started studying PVS, there were several scales for visual assessment available, even though few scales have been used in >1 study, probably because most of these scales had great benefits, but also drawbacks. Most of the previous scales were only concerned with the PVS number and used a fixed diameter of 224 or 3 mm19 to separate PVS from lacunes, even though PVS can sometimes grow very large.25 For instance, one scale measured the mean diameters of all PVS in 1 axial section at the level of the cella media of the lateral ventricles, which means that the scale mainly evaluated the frontal and parietal white matter.5 This scale left out the basal ganglia, where many PVS are located. In another scale, the PVS both along the lenticulostriate arteries (in the basal ganglia) and in the high convexities were assessed.4 The scale was based on the width of PVS but was also given 1 point if there were >4 PVS in each region. Thus, the scale did not really separate the diameter from the number of PVS. The third scale rated the number of PVS only in the most affected hemisphere but combined the basal ganglia with the centrum semiovale and rated only the hippocampus separately.6 Thus, this scale could not differentiate PVS in the basal ganglia from those in the centrum semiovale. This drawback was overcome in a later study.19
The scale by Patankar et al7 rated the number of PVS in 4 different regions, which makes this scale very appealing; thus, it has been used by other groups.26,27 However, this scale has a great disadvantage in a complicated grading, where each region has a different grading scale with 2 different schemes for the basal ganglia. Because we aimed to develop a rating scale that was easy to use, we decided to use the grades 0–3 for both the diameter and number of PVS. Our rating scale is in line with the requirements of the STRIVE in terms of rating both the number and size in different locations.12
We found a slight increase in the global score for PVS number with advancing age, which is in accordance with most other studies.4,8,9,13,28⇓⇓–31 Our study sample was relatively healthy,20 which might partly explain a smaller increase with age than expected, because PVS are presumably correlated with the degree of cerebral small-vessel disease and amyloid deposition with increasing age.17,18 We did not find a sex difference in the distribution of PVS, even though it has been reported in earlier studies11,13,16; notably, one of these studies comprised a very large population-based sample (n = ∼1800), in which men were found to have more PVS in the basal ganglia than women,13 while another large-scale study of patients with ischemic stroke (n = 1090) found that men had more PVS in the white matter than women, but there was no sex difference in the distribution of PVS in the basal ganglia.11 The discrepancies in the findings may be partly attributable to the differences in characteristics of the study populations.
In our sample, we found very few PVS >4 mm (1.1% of all subjects or 0.4% of all the examined brain regions), which is in accordance with the traditional view that PVS should be <5 mm.32 In addition, our data showed rather limited variation in the size of PVS by age. This appears to be contradictory to a clinically based study, which showed a strong correlation of the width of the PVS with increasing age.4 However, because the scale in that study yielded a higher score if there were many PVS, it might have reflected an increasing number, rather than size, of PVS with age. Given that the width of PVS has been correlated with advancing age,4 it might still be important to also include the diameter when assessing PVS.
A higher global PVS score was correlated with a greater burden of WMH in lobar regions and the basal ganglia, which supports the view that PVS, along with WMH, are markers for cerebral small-vessel disease.9,19,33 More interesting, we found a correlation between the PVS score in the basal ganglia and WMH load not only in the same region but also in lobar areas, while we did not find any correlation between the lobar PVS score and WMH load either in the lobar areas or in the basal ganglia. This is in line with the view that PVS, especially in the basal ganglia, are caused mainly by hypertensive arteriopathy, like WMH, while lobar PVS are mainly due to cerebral amyloid angiopathy or amyloid accumulation in the brain with normal aging.10,30
A major strength of our study is the large sample from the general population. To date, only very few studies have had such a large sample of community dwellers.4,13,29 In addition, because our scale is based on counting the number and measuring the diameter of PVS, it can be used on different MR imaging sequences, as opposed to the visual impression–based rating scales. Furthermore, the regionalization in the assessments of both PVS and WMH is of relevance when studying their correlations with vascular risk factors and cognitive aging.
Our study also has limitations. First, we used diameter to measure the severity of PVS, which may be less precise than the direct measurement of PVS volume. Indeed, semiautomatic approaches for assessing PVS volume are being developed,16 though current volumetric methods do not cover the entire brain or work as fast as visual rating approaches.34,35 Second, our scale includes 2 parameters (number and size) of PVS in 14 regions, which is rather time-consuming (on average 6 minutes per examination). This means that our study has an advantage for research purposes. For visual assessment of several imaging markers (eg, WMH, microbleeds, and brain atrophy) or for clinical use, however, the scale of Potter et al9 may be easier to use for rating PVS in the most important regions such as the basal ganglia and centrum semiovale.
Conclusions
We describe a new semiquantitative scale for visual assessment of PVS that considers the number and diameter of PVS as well as location. This rating scale has excellent intra- and interrater reliability. Using this scale, we found that the global number of PVS increased with age and correlated with increasing load of WMH in lobar and deep white matter regions. This rating scale will be useful for future research on PVS, especially regarding risk factors and functional consequences of PVS by region. We plan to further investigate vascular risk factors and cognitive decline associated with global and regional PVS. A potential implication of linking global and regional PVS to functional outcomes is that the presence of visible PVS may be important to include in clinical reports on brain MR imaging examinations in the future, as we currently do for WMH in our clinical reports.
Acknowledgments
We are grateful to all the SNAC-K participants and to our colleagues in the SNAC-K group for their collaboration in data collection and management. We thank Dr Heather Martin from the Department of Neuroradiology, Karolinska University Hospital, Stockholm, Sweden, for careful editing of our manuscript.
Footnotes
Disclosures: Rui Wang—RELATED: Grant: The Swedish Ministry of Health and Social Affairs, the participating County Councils and Municipalities, and the Swedish Research Council. Chengxuan Qui—RELATED: Grant: The Swedish Ministry of Health and Social Affairs, the participating County Councils and Municipalities, the Swedish Research Council, and the Swedish Research Council for Health, Working Life and Welfare.* *Money paid to the institution.
SNAC-K was supported by the Swedish Ministry of Health and Social Affairs, the Stockholm County Council, Stockholm Municipality, and the Swedish Research Council. This work was further supported, in part, by grants from the Swedish Research Council (grant Nos. 2015-02531 and 2016-06658); the Swedish Research Council for Health, Working Life and Welfare (grant No. 2014-01382); and the Karolinska Institutet, Stockholm, Sweden.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received August 16, 2016.
- Accepted after revision September 1, 2017.
- © 2018 by American Journal of Neuroradiology