Abstract
BACKGROUND AND PURPOSE: The endovascular treatment of aneurysms located at or distal to the circle of Willis and not amenable to coiling remains a challenge. We report our experience with flow-diversion treatment using low-profile braided stents as a stent monotherapy procedure for treating distally located very small or uncoilable aneurysms.
MATERIALS AND METHODS: We retrospectively reviewed our data bases to identify patients with aneurysms located at or distal to the circle of Willis who were treated with stent monotherapy using low-profile braided stents. The immediate and follow-up angiographic findings and clinical status of the patients were assessed.
RESULTS: Twenty aneurysms in 19 patients were included in the study. The mean size of the aneurysms was 4.7 ± 2.4 mm. Patients were treated via telescopic implantation of 2 stents for 11 aneurysms; single-stent placement was used for the remaining aneurysms. The technical success rate was 95%. We observed a technical complication in 1 case (5.3%) and a late ischemic event in another (5.3%). The final angiographies during a mean follow-up of 14.7 months showed complete aneurysm occlusion in 73.7%. The complete occlusion rate of the aneurysms treated with telescopic stent placement was 81.8%. The modified Rankin scale scores of all patients at the last follow-up were between 0 and 2.
CONCLUSIONS: Flow diversion with low-profile braided stents as a stent monotherapy procedure for very small or uncoilable intracranial aneurysms located at or beyond the circle of Willis is a promising, relatively safe, and durable endovascular procedure.
ABBREVIATION:
- OKM
- O'Kelly-Marotta grading scale
In the past decade, several self-expandable stents dedicated to intracranial use have been introduced to treat wide-neck and complex aneurysms previously not amenable to coiling.1⇓⇓–4 Stents create a mechanical scaffold, which prevents coil protrusion into the parent artery. In addition to this mechanical scaffolding effect, the implantation of stents also produces hemodynamic and biologic effects in the parent arteries that promote aneurysm occlusion. Stent deployment across the orifice of an aneurysm redirects the blood flow in the parent artery to decrease hemodynamic stress, which facilitates thrombosis in the aneurysmal sac.5 Furthermore, stents induce neointimal proliferation in the parent artery, which eventually leads to healing of the aneurysm neck.6 The hemodynamic and biologic effects of stents promote the progressive occlusion of partially coiled aneurysms and impede their recanalization.
On the basis of the hemodynamic and biologic effects of stents, flow-diversion treatment as a stent monotherapy procedure using conventional stents has been proposed for the endovascular treatment of intracranial aneurysms not amenable to coiling or alternative open surgical procedures.7 Flow diversion as a stent monotherapy procedure consists of the implantation of a self-expandable stent or stents across the neck of an aneurysm, without coiling the aneurysm sac. A limited number of previous case series reported the application of stent monotherapy with balloon-expandable or conventional self-expandable stents and focused on the treatment of aneurysms located proximal to the circle of Willis.7⇓⇓–10
Low-profile braided stents have been recently introduced to treat aneurysms located at small-sized, distal parent arteries. Low-profile intracranial stents can be deployed into arteries with diameters between 1.5 and 3.5 mm, and they can be delivered through microcatheters with an internal diameter of 0.0165 inches, which allows easier navigation in small-sized, delicate vessels.11 In this report, we present our experience with flow-diversion treatment as a stent monotherapy procedure for treating very small or uncoilable intracranial aneurysms located at or beyond the circle of Willis using low-profile braided stents. In this retrospective study, we investigated the feasibility, efficacy, and midterm durability of the stent monotherapy procedure with LEO Baby stents (Balt, Montmorency, France).
Materials and Methods
After approval of the study by the institutional ethics committee, we retrospectively reviewed our data base records to identify patients with intracranial aneurysms located at or distal to the circle of Willis who were treated with the stent monotherapy procedure using low-profile stents. In all cases, the decision regarding the most appropriate method of treatment was made by a multidisciplinary neurovascular team. Flow diversion with low-profile braided stents as a stent monotherapy procedure was performed to treat patients with very small aneurysms (<3 mm) located at or distal to the circle of Willis, distal aneurysms with a complex morphology that impeded the application of the conventional endovascular techniques such as balloon remodeling or stent-assisted coiling, and distal aneurysms from which important branches or perforators arose.
The review of our data base records revealed 19 patients with 28 aneurysms who underwent the stent monotherapy procedure to treat intracranial aneurysms. There were 15 female (79%) and 4 male patients, with a mean age of 46.6 ± 17.9 years (range, 7–71 years). Twenty aneurysms in 19 patients were included in this study. Eight aneurysms treated with other endovascular techniques or clipping were excluded. All except 1 of the aneurysms reported in this study were unruptured. One patient with an anterior cerebral artery aneurysm was referred to our center for endovascular surgery 3 weeks after the rupture of the aneurysm. Another patient with an anterior cerebral artery aneurysm had a history of subarachnoid hemorrhage caused by the rupture of a contralateral middle cerebral artery aneurysm, which was treated by clipping at that time. Two aneurysms had been previously coiled and then became recanalized during the follow-up assessments. Eighteen of the 20 aneurysms had not received prior treatment. The mean dome size of the aneurysms was 4.7 ± 2.4 mm (range, 1.5–9 mm). Five aneurysms were in the A1–2 segments of the anterior cerebral artery, 4 aneurysms were in the posterior-inferior cerebellar artery, 3 aneurysms were in the pericallosal segment of the anterior cerebral artery, 3 aneurysms were in the posterior cerebral artery, 3 aneurysms were in the M1 segment of the MCA, and 2 aneurysms were in the bifurcation of the MCA. Three aneurysms had a complex morphology, and 17 aneurysms were saccular.
The preoperative clinical status of patients was evaluated with the modified Rankin Scale. The preoperative mRS scores of 17 patients were all zero. Two patients with a history of subarachnoid hemorrhage had a preoperative mRS score of 2.
Endovascular Procedure
In all cases, the endovascular procedure consisted of the implantation of a low-profile, self-expandable stent or stents covering the neck of the aneurysm, without coiling of the aneurysm. The patients with unruptured aneurysms received 75 mg of clopidogrel and 300 mg of aspirin daily for at least 5 days before the procedure. In the patient with a recently ruptured aneurysm, antiplatelet therapy was initiated 12 hours before the endovascular procedure with a loading dose of 300 mg of aspirin and 600 mg of clopidogrel. A 6F guiding catheter (Envoy; Codman & Shurtleff, Raynham, Massachusetts) or a 6F guiding sheath (Neuron Max; Penumbra, Alameda, California) was placed into the target artery (the internal carotid artery or the proximal vertebral artery) at the beginning of the procedure. A microcatheter (Echelon 10, Covidien, Irvine, California; or Vasco 10, Balt) for stent delivery was positioned in the parent artery distal to the neck of the aneurysm with the guidance of a 0.014-inch microguidewire (Transend, Stryker, Kalamazoo, Michigan; Traxcess, MicroVention, Tustin, California; Hybrid, Balt). A low-profile braided stent (LEO Baby plus; Balt) was deployed into the parent artery to cover the neck of the aneurysm in all cases.
After the deployment of the stent, control angiography was performed to assess the opening and the apposition of the stent. In some cases, an additional stent was deployed, overlapping the initial stent. In these cases, the stent delivery microcatheter was navigated through the first deployed stent, and the second stent was telescopically deployed inside the initial stent. If there was no sign of any complication on the control angiograms, the stent delivery wire was removed and the procedure was completed. Postprocedural dual antiplatelet treatment, including 75 mg/day of clopidogrel and 300 mg/day of aspirin, was continued for 3–6 months. Thereafter, the dual antiplatelet therapy was switched to aspirin.
All technical and clinical complications were recorded. Complications were defined as periprocedural if they occurred during the procedure or within 48 hours following completion of the procedure. Clinical complications that occurred between 48 hours and 14 days were defined as early postprocedural, while those occurring after 2 weeks were defined as late postprocedural complications. The neurologic status of patients was recorded at discharge from the hospital using the mRS score.
Follow-Up Assessment
An immediate postprocedural control angiography was performed at the end of the procedure to evaluate the patency of the stents, the filling status of the aneurysm, and the patency of side branches and perforators. The filling status of the aneurysms was evaluated with the O'Kelly-Marotta (OKM) grading scale.12 The degree of aneurysm filling was graded between A and D (A = complete filling, B = incomplete filling, C = entry remnant, D = total occlusion). The degree of stasis in the aneurysm sac was scaled between 1 and 3 (1 = arterial, 2 = capillary, 3 = venous). Follow-up MR angiography was performed at 3–4 months. The first digital subtraction angiography follow-up was performed at 3–6 months. The second examination was performed at 9–12 months. The third follow-up angiography was performed at 24–36 months, depending on the findings of the second follow-up angiography. The neurologic status of patients was assessed with the mRS score at each follow-up.
Results
Thirty-one stents were deployed to treat 20 aneurysms. The telescopic implantation of double stents was performed to treat 11 aneurysms. Single stents were deployed for the remaining 9 aneurysms (Fig 1). The technical success rate of the procedure was 95% (19 of 20 aneurysms) (Fig 2). A technical complication developed in 1 patient with a dissecting/fusiform PICA aneurysm, which resulted in the failure of the procedure. The parent artery (the left PICA) in this patient had stenotic and extremely tortuous segments. After the deployment of the stent, the immediate control angiography revealed an incomplete opening of the stent in the stenotic/tortuous segment and the subsequent development of acute and complete thrombosis of the stent. The thrombosed stent was then retrieved with a microcatheter and microsnare to restore blood flow in the PICA. This patient awoke without any neurologic deficits. The deployment of stents in all the other patients was uneventful.
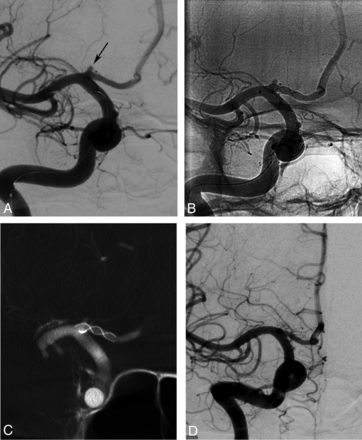
Procedural and follow-up angiograms of a 28-year-old female patient with a history of subarachnoid hemorrhage due to rupture of a contralateral MCA bifurcation aneurysm. A, A preprocedural DSA image shows a 1.5-mm saccular aneurysm (arrow) located in the A1 segment of the right anterior cerebral artery. B, A nonsubtracted angiography image obtained during the procedure demonstrates the successful implantation of a stent in the A1 segment of the anterior cerebral artery, covering the neck of the aneurysm. This image also shows the filling of the aneurysm (OKM grade A). C, A VasoCT image (Philips Healthcare, Best, the Netherlands) obtained immediately after stent deployment reveals the full apposition of the stent to the parent artery walls. D, The 10-month follow-up DSA image shows complete occlusion of the aneurysm (OKM grade D).
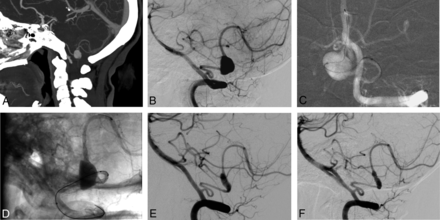
Preprocedural CT and procedural and follow-up angiograms of a 39-year-old female patient with a distal PICA aneurysm. A, CT angiography reconstructed on a sagittal projection shows a saccular aneurysm in the PICA. B, A preprocedural DSA image depicts a 7-mm saccular aneurysm located in the tonsillomedullary segment of the left PICA. C, A roadmap DSA image obtained during the procedure shows the catheterization of the distal PICA for stent placement. This image also demonstrates that the aneurysm has a very complex morphology without a definable neck. D, A nonsubtracted angiography image shows the deployment of the stent into the PICA. Please note the compaction and expansion of the stent across the orifice of the aneurysm. E, A 12-month follow-up DSA image reveals the reconstruction of the parent artery and no aneurysm filling. F, The 24-month follow-up DSA image confirms the stable occlusion of the aneurysm.
The immediate control angiography images revealed complete occlusion of 1 aneurysm (OKM grade D) (5%), stagnated partial filling of 3 aneurysms (OKM grade B2–3) (15%), stagnated filling of 11 aneurysms (OKM grade A2–3) (55%), and complete filling of 5 aneurysms, including the case in which the stent was retrieved due to acute thrombosis (OKM grade A1) (25%) (Fig 3).
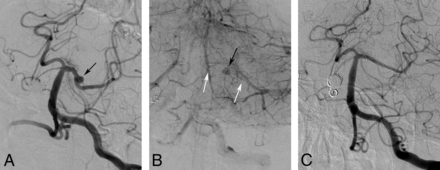
Procedural and follow-up DSA images of a 62-year-old female patient with a small posterior cerebral artery aneurysm. A, A preprocedural DSA image obtained in the left anterior oblique projection reveals a 2.5-mm saccular aneurysm (arrow) located in the left posterior cerebral artery. B, The venous phase of the DSA image obtained immediately after stent deployment shows the stagnation of injected contrast medium in the aneurysm sac (black arrow) and the deployed stent (white arrows) in the left posterior cerebral artery. C, The 7-month follow-up DSA image demonstrates total occlusion of the aneurysm (OKM grade D) and a mild degree of in-stent stenosis.
Complications
No mortality occurred during this study. Complications, including the asymptomatic technical complication, developed in 2 patients (10.5%). The mRS scores at discharge were equal to the preoperative scores for all patients. We observed a late postprocedural complication in a patient with an MCA aneurysm treated by the telescopic implantation of 2 stents (5.3%). This patient developed monoparesis in his right (contralateral) leg 2 months after the cessation of clopidogrel at the 6-month follow-up. His cranial MR image revealed a small lacunar infarct in the left basal ganglia extending to the genu of the internal capsule. We prolonged the dual antiplatelet treatment in this case, and his symptoms regressed completely within weeks. His final mRS score at the last follow-up was zero.
Follow-Up Assessments
Follow-up imaging was performed for 19 aneurysms in 18 patients (94.7%). The mean duration of follow-up was 14.7 ± 8.5 months (range, 4–36 months). The follow-up imaging revealed an OKM grade D in 14 of 19 aneurysms (73.7%), OKM C2–3 grade filling in 2 aneurysms (10.5%), OKM B2–3 filling in 2 aneurysms (10.5%), and OKM grade A3 filling in 1 aneurysm (5.3%). None of the aneurysms ruptured during the follow-up period. The last follow-up of 11 aneurysms treated with telescopic stent placement revealed total occlusion (OKM grade D) of 9 aneurysms (81.8%) (Fig 4). The last follow-up angiography assessments of the aneurysms treated with a single stent revealed a total occlusion rate of 62.5%.
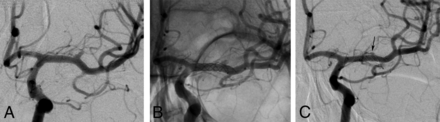
Procedural and follow-up angiograms of a 48-year-old female patient with an MCA aneurysm. A, A preprocedural DSA image reveals a 2.5-mm saccular aneurysm located in the M1 segment of the left MCA. This image also shows a lenticulostriate artery arising from the aneurysm. B, A nonsubtracted angiogram recorded during the procedure demonstrates the telescopic implantation of 2 low-profile stents into the left M1 segment. C, The 14-month follow-up DSA image shows total occlusion of the aneurysm (OKM grade D) and the reconstruction of the lenticulostriate artery (arrow), which previously arose from the aneurysm.
We observed in-stent stenosis in the first follow-up angiography examinations of 5 patients (27.8%). In 3 of them, the aneurysms had been treated by the telescopic implantation of double stents. The second follow-up angiography examinations demonstrated regression of the in-stent stenosis in 3 patients. The in-stent stenosis was mild (<50%) and remained asymptomatic in all cases. Therefore, no intervention was performed to treat the in-stent stenosis.
The mRS score at the last follow-up evaluation was zero in 16 of 18 patients. The mRS scores of 2 patients with preprocedural scores of 2 did not change until their last follow-up.
Discussion
The endovascular treatment of distally located, very small-sized aneurysms is a challenge for interventional neuroradiologists. The risk of intraprocedural rupture is significantly high during the coiling of very small aneurysms.13,14 The microcatheterization of small aneurysms is very difficult, and microcatheter or microguidewire maneuvers can cause rupture. Furthermore, because of the limited space inside the aneurysm sac, coiling itself can lead to the rupture of a small aneurysm. We performed the stent monotherapy technique with a flow-diversion strategy to treat very small aneurysms located at or distal to the circle of Willis. The stent monotherapy procedure is a safe alternative endovascular method for treating distally located, very small-sized aneurysms. The lack of aneurysm catheterization nearly eliminates the risk of intraprocedural rupture of very small aneurysms during stent monotherapy.
The principle of stent monotherapy for intracranial aneurysms is based on flow diversion and the biologic effects of stents. Aneurysm flow velocity and wall shear stress are important hemodynamic parameters associated with the growth and rupture of intracranial aneurysms.15⇓⇓⇓–19 The placement of a stent or stents across the neck of an aneurysm redirects the blood flow and decreases intra-aneurysmal flow velocity by disturbing the inflow. The reduced aneurysmal flow induces stasis and consequently thrombosis of the aneurysm.5 Tateshima et al19 investigated the alterations in intra-aneurysmal hemodynamics with the placement of high-porosity open-cell stents across the necks of aneurysms and found that the placement of a single Neuroform stent (Stryker Neurovascular) reduced the intra-aneurysmal flow velocity by 22%–64%. In another study, Wang et al20 assessed the hemodynamic changes inside aneurysms induced by the implantation of conventional stents and found that the velocity of the jet flow entering the aneurysm and wall shear stresses on the aneurysm were significantly reduced by the placement of a stent.20 These authors suggested that the hemodynamic changes induced by stents promote thrombus formation inside the aneurysm and reduce the risk of aneurysm growth and rupture. Blankena et al21 studied the correlation between wall shear stress and wall thickness of aneurysms with 7T MR imaging. This group found an inverse correlation between wall shear stress and aneurysm wall thickness. High wall shear stress might be associated with the process of aneurysm wall remodeling, which causes thinning of the aneurysm wall.
The results of previous experimental studies suggest that the hemodynamic changes induced by the placement of stents across the aneurysm neck promote thrombus formation inside the aneurysm, reducing the risks of aneurysm growth and rupture. Fiorella et al7 reported the results of 10 patients whose ruptured aneurysms were treated with stent monotherapy by the implantation of open-cell stents. Even though the flow-diversion capacity of high-porosity open-cell stents is relatively low, in their results during a 12-month follow-up period, Fiorella et al observed complete occlusion in 5 (50%) patients and near-total occlusion in 4 (40%). Kim and Ko10 treated 8 patients with ruptured, small intracranial aneurysms with stent placement alone, without coiling. Follow-up angiographies revealed total aneurysm occlusions in 3 patients (37.5%) and size reduction in 3 patients (37.5%). These investigators did not observe any growth or rupture of the aneurysms during the follow-up periods ranging from 6 to 34 months. Zenteno et al9 reported the results of performing the single-stent placement technique to treat posterior circulation aneurysms.
One-year angiographic follow-up examinations revealed a total aneurysm occlusion rate of 80% in this study. The results of our study support the findings of previous experimental and clinical studies. We observed total occlusion in 73.7% of the aneurysms during the mean follow-up of 14.7 months. Furthermore, 21.0% of the aneurysms showed a reduction in size and stasis in the sac during the follow-up period. Additionally, none of the aneurysms showed growth, and we did not observe any aneurysm rupture. Therefore, the results of our study indicate that flow diversion with low-profile braided stents as a stent monotherapy is an effective endovascular technique for the treatment of very small or uncoilable intracranial aneurysms located at or distal to the circle of Willis.
LEO Baby stents are self-expanding stents that are constructed of braided metal filaments. They have a relatively smaller cell size (approximately 0.9 mm) and a higher metal coverage ratio than other conventional self-expandable stents. The porosity of LEO Baby stents at their nominal diameter is approximately 83%. Bouillot et al22 studied the flow modifications induced by conventional self-expandable and low-porosity flow-diverter stents and found that conventional, intermediate-porosity braided stents could provide flow-change patterns like those provided by low-porosity flow-diverter stents. The relatively high mesh density of LEO Baby stents provides a relatively high flow-diversion capacity and allows these stents to act as mini flow diverters in appropriate cases. Moreover, it is possible to further decrease the porosity of braided stents during their deployment. When an axial force is applied to braided stents during deployment, the stents can expand along vessel segments with a diameter larger than the nominal diameter of the stent. The expansion of the stent beyond its nominal diameter in unconstrained segments causes a significant reduction in the porosity.23
Therefore, it is possible to compress braided stents in unconstrained segments to decrease the porosity and increase the flow-reconstruction capacity. We used this feature of LEO Baby stents as a strategy to increase the flow-diversion capacity of the stents deployed to treat the wide-neck or dissecting/fusiform aneurysms. During the deployment of the stents, we attempted to compress the stents by applying an axial force with a delivery microcatheter and a delivery wire. Our second strategy to increase the metal density over the neck of the aneurysm was the telescopic deployment of 2 stents. We performed telescopic stent placement in the treatment of 11 of 20 aneurysms. In an experimental study, Cantón et al24 showed that the sequential placement of multiple stents across the aneurysm neck progressively decreases the hemodynamic stress on the aneurysm. Kim et al25 used a computational fluid dynamics method to quantify the hemodynamic changes achieved by the placement of multiple stents. Kim et al found that the placement of 2 stents caused a significant reduction in wall shear stress and an increase in the turnover time of intra-aneurysmal flow. Our findings support the results of these experimental studies. In our study, the rate of total occlusion for the aneurysms treated with telescopic stent placement was 81.8%, which is higher than the follow-up results of single-stented aneurysms. This finding indicates that the telescopic implantation of a second stent in appropriate cases would increase the flow-diversion capacity of the stent monotherapy procedure.
Low-porosity stents dedicated to flow diversion have a much higher metal density compared with conventional self-expandable stents. The high metal density of flow-diverter stents might induce faster aneurysm exclusion. However, the risk of side branch or perforator occlusion would also increase with a higher metal density. The patency of covered side branches and perforators is a major debate in the recent use of flow diverters for the treatment of distally located intracranial aneurysms. Gawlitza et al26 treated 18 aneurysms located beyond the circle of Willis with the implantation of flow-diverter stents, and 17.6% of their patients developed symptomatic ischemic lesions in the territories of the perforators covered by the stents. Furthermore, the follow-up MR imaging examinations revealed asymptomatic lacunar ischemic lesions in 29.4% of the cases. In another study, Pistocchi et al27 treated 30 aneurysms located beyond the circle of Willis with the implantation of flow-diverter stents. These investigators observed the total occlusion of jailed side branches in 38.1% of the cases and restricted flow in 23.8% of the cases.
Because of the potentially high perforator or side branch occlusion risk, we preferred to not use low-porosity flow-diverter stents in our patients. No permanent morbidity was observed in our patients. We observed a symptomatic thromboembolic complication in only 1 patient who developed a lacunar infarction in the territory of the perforators covered by the telescopic stents. The symptoms of this patient regressed completely during the follow-up period. Furthermore, the mRS scores at the last follow-up examinations did not differ from the preprocedural scores for any of the patients.
Another disadvantage of flow-diverter stents in the treatment of distal intracranial aneurysms is the current necessity of catheterizing small-sized intracranial arteries with larger and stiffer microcatheters with internal diameters of 0.021–0.027 inches. The catheterization of distal and small-sized vessels with these large, stiff microcatheters could be technically difficult and traumatic. Low-profile, self-expandable stents are deliverable through low-caliber microcatheters with an internal diameter of 0.0165 inches. Therefore, low-profile stents allow easier catheterization and navigation in small-sized, delicate vessels and enable safer stent placement during the treatment of distal aneurysms. In our patients, some of the aneurysms were in very small-sized and extremely tortuous parent arteries, which could be safely catheterized with low-caliber delivery microcatheters.
In flow-diversion treatment for intracranial aneurysms, the hemodynamic alterations provided by the stent struts initiate the formation of a thrombus inside the aneurysm. However, the final steps necessary to achieve total and durable aneurysm occlusion depend on the cellular processes induced by the stent implantation.28 These cellular events start with the adherence of inflammatory cells to the intersections of the stent struts. Then, endothelialization that begins along the parent artery proceeds over the aneurysm neck. The complete endothelialization of the stent struts over the neck is associated with total occlusion of the aneurysm. Because the endothelial cells that grow over the stent struts are derived from the adjacent parent artery, the full apposition of the stent to the parent artery wall is critical for the achievement of a total aneurysm occlusion.29 LEO Baby stents have a braided, sliding strut design, providing a high degree of conformability and allowing good wall apposition even in tortuous vessels, which may have facilitated the development of total occlusion and the regression of the aneurysms in our study.
The in-stent stenosis incidence at the first follow-up DSAs was relatively high compared with the results of previous studies.1,7 However, most (60%) regressed during follow-up, and all patients remained asymptomatic. The incidence of in-stent stenosis among patients treated with telescopic (27.3%) and single stent placement (28.6%) was similar. Therefore, it seems to be a reversible reaction of endothelium induced by the stent implantation.30 When we consider that the mechanism of aneurysm occlusion following flow-diversion treatment is based on the stent endothelialization, in-stent stenosis may be a consequence of an endothelial healing process that is induced by stent implantation.1,28
There are some limitations to the current study. First, this study was a nonrandomized, retrospective study. Therefore, there was no control group of alternative endovascular or open surgical treatment methods with which to compare the results. The decisions for the most appropriate endovascular method of treatment were made by the neurovascular teams in each patient. Therefore, the effects of patient selection bias cannot be excluded from the results. Finally, the study population was relatively small.
Conclusions
Flow diversion with low-profile braided stents as a stent monotherapy is a promising, relatively safe, and durable endovascular procedure to treat very small or uncoilable intracranial aneurysms located at or beyond the circle of Willis. Flow diversion with low-profile braided stents may be considered an alternative option to treat distal intracranial aneurysms not amenable to conventional endovascular techniques. Larger series with longer follow-up are necessary to define the long-term durability of this treatment.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 31, 2017.
- Accepted after revision July 3, 2017.
- © 2017 by American Journal of Neuroradiology