Abstract
SUMMARY: Visual hallucinations are relatively uncommon presentations in medical and psychiatric clinics, where they are generally regarded as a marker of possible underlying “organic” brain disease. Thus, patients with visual hallucinations are often referred for imaging of the brain. This article presents a pragmatic approach for the radiologist reviewing such imaging. Because conditions that can present with visual hallucinations are legion, a familiarity with the features of the hallucinations themselves, which can serve as clues to the underlying cause, can be helpful in interpreting such cases. We consider the nature of visual hallucinations and the mechanisms underlying their formation. We then provide a framework to guide the search for their cause, first in terms of focal lesions along the visual pathway and then global conditions affecting >1 region.
ABBREVIATIONS:
- CJD
- Creutzfeldt-Jakob disease
- VH
- visual hallucination
The presentation of visual hallucinations (VHs) to general medical and psychiatric clinics often triggers a search for underlying “organic” brain disease and a referral for imaging of the brain, first with CT and then MR imaging. If the findings are interpreted as normal, patients who in actuality have underlying organic disease can have delays in diagnosis and prolonged inappropriate management. Therefore, it behooves the reporting radiologist to be familiar with visual hallucinations and the possible causes thereof.
The organic causes of VHs represent a veritable Augean stable of pathologies, ranging widely in etiology and location within the brain (Table 1). Although in some instances, a focal defined lesion can lead to VHs (eg, an occipital lobe cavernoma), pathology can also affect large or multiple areas simultaneously (eg, posterior cortical atrophy or Creutzfeldt-Jakob disease [CJD]). When one reviews scans of patients with VHs, it is important to assess not only each part of the visual system but also more diffuse, global, or multiregional pathologies. We have pragmatically divided this article into focal and global causes based simply on localization rather than on a clear understanding of the pathophysiology of VHs. We briefly consider the nature of hallucinations and clues in the clinical context on the request form. We then consider mechanisms underlying the formation of VHs to guide the search for their cause. We suggest looking first at focal lesions along the visual pathway and then conditions affecting >1 region. Only when no lesion is found and in the absence of other organic clinical features should functional causes then be considered.
Types of Visual Hallucinations
A hallucination is a “percept without object,”1 “a sensory perception that has the compelling sense of reality but that occurs without stimulation of the relevant sensory organ.”2 Hallucinations are distinguished from the following: 1) distortions, in which the real objects are perceived as changed in some way; 2) illusions, in which the perception of real objects is transformed in size (micropsia or macropsia), shape (metamorphopsia), or color (dyschromasia) or into other objects; or 3) pseudohallucinations, which arise from vivid inner mental experience and can often be recognized as such. Although hallucinations are experienced as real, patients experiencing them have varying degrees of insight into the nature of their experiences, which engender varying responses, from indifference to marked distress. Hallucinations vary in content and complexity and occur in every sensory technique: Visual hallucinations are commonly linked to underlying organic etiology but also occur frequently in psychotic states, though half as commonly as auditory hallucinations. Olfactory, tactile, and gustatory hallucinations occur less often and are seen in a variety of both psychiatric and organic conditions. The use of the term “organic” here is by convention and should not be taken to imply an absence of brain dysfunction in psychiatric illness.3
The content of visual hallucinations can offer some clue as to their origin (Table 1) and may relate to the mechanism of production.
Type of hallucination
Simple Visual Hallucinations.
Brief, stereotyped unformed flashes of light and color or indistinct forms may reflect stimulation or irritation of primary visual areas, for example by tumor, migraine, or focal epileptogenic lesions.
Complex Visual Hallucinations.
In contrast, complex visual hallucinations suggest disruption to the wider visual system4 and include branching or tessellated patterns, individuals or crowds of people, animals, and complex scenes often associated with sensory distortions. Lilliputian hallucinations, classically seen in alcohol withdrawal and delirium, are complex VHs consisting of miniature people in lines or groups performing strange actions and eliciting curiosity or wonder. Complex VHs due to psychiatric disturbance, delirium, or intoxication/withdrawal are often perceived as real and frightening, while those seen in peduncular hallucinosis or the Charles Bonnet syndrome may provoke indifference, and insight into the nature of the experience as unreal may be preserved. Associated symptoms such as headaches or focal seizures may help point toward a specific etiology, as may the presence of associated deteriorating cognitive function, focal neurologic symptoms, or psychiatric symptoms (Table 2).
Associated symptoms
Visual Pathway and Mechanisms of Disruption
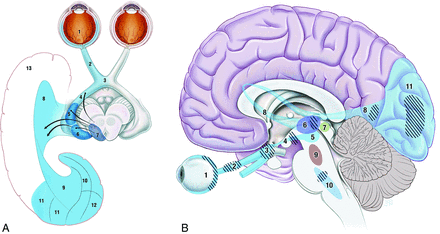
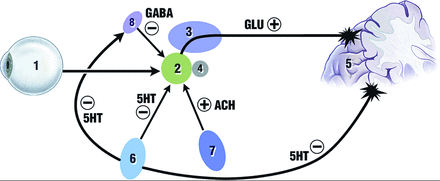
The anatomy of the primary visual pathway is well-described: Information from the retina passes along the optic nerve, chiasm, and tract to the lateral geniculate nucleus in the thalamus and then to the optic radiation through the temporal lobe to the primary and secondary visual cortices (Fig 1). The flow of visual information is modulated by ascending input from the pedunculopontine and parabrachial nuclei and raphe nuclei via the superior colliculi (Fig 2) and involves the cholinergic, GABAergic, and glutamateric systems (Fig 2).
Visual pathways. A, Retino-geniculo-calcarine tract. Optical information from the retina (1) passes along the optic nerve (2) through the optic chiasm (3) and optic tract (4) into the lateral geniculate nucleus of the thalamus (5), where it receives input from the superior colliculus (7) via the pulvinar (6) and then traverses the optic radiation (8 and 9) through the temporal lobe (13) into the visual cortex (10–12). B, Intersection of ascending pathways. Optical information in the retino-geniculo-calcarine tract (1–8 and 11) is modulated by ascending input from the pedunculopontine and parabrachial nuclei (9) and raphe nuclei (10) via the superior colliculus (7). Hashed areas show regions where interruptions are known to produce visual hallucinations: in the retino-geniculo-calcarine tract via deafferentation, in the thalamus through reducing signal-to-noise ratio, and in the ascending pathways via removal of inhibitory control. Reproduced with permission from Dr. Ramon Mocellin.
Neurochemistry of vision. Input from the retina (1) reaches the lateral geniculate nucleus of the thalamus (2). This structure and the adjacent pulvinar of the thalamus (3), an accessory visual structure that may act to filter out eye-movement “noise,” act as a junction between retino-geniculo-calcarine and ascending brain stem circuits, receiving inhibitory serotonergic input from the raphe nuclei (6) and excitatory cholinergic input from the pedunculopontine and parabrachial nuclei (7). The reticular nucleus of the thalamus (8) also provides inhibitory GABAergic innervation to the geniculate, which is itself modulated by the same ascending cholinergic and serotonergic input. The glutamatergic excitatory circuits from the geniculate to the occipital cortex (5) are also modulated by the superior colliculus (4). Reproduced with permission from Dr. Ramon Mocellin.
Interruptions to this system at any point, either in the primary direct pathway or in its ascending modulatory projections, may lead to visual hallucinations. One series by Braun et al5 suggested that the occipital and occipitotemporal regions were the most commonly implicated cortical regions, and the midbrain, cerebral peduncles, pons, and thalamus, the usual subcortical regions. A search for focal lesions on MR imaging should progress with this pathway in mind.
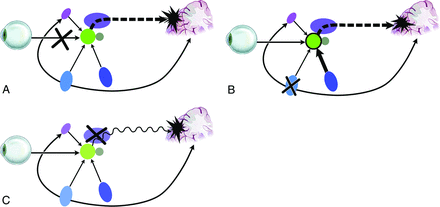
The exact mechanisms underlying these hallucinations remain unclear but may involve cortical release or deafferentation phenomena (Fig 3A)6 and/or the disinhibition of projections from ascending pathways or the intact nearby visual cortex. Disruption of ascending input, for example at the lateral geniculate nucleus, may lead to aberrant projections forward to the visual cortex (Fig 3B) or a loss of central sensory filtering function and degradation of signal to noise (Fig 3C).
Possible mechanisms of visual hallucinations. A, Deafferentation: lesions responsible for pathway complex visual hallucination in which deafferentation from ocular input results in “release” activity in the cortex. B, Disinhibition: lesions responsible for ascending complex visual hallucinations in which a loss of ascending inhibition to the geniculate results in a hyperexcited geniculate and excess glutamatergic activity in the optic radiation, with resultant poor-quality signal to the cortex. C, Central: lesions producing central complex visual hallucinations in which damage to the geniculate may again “deafferent” the striate cortex and lesions to the pulvinar of the thalamus may reduce the signal-to-noise ratio of cortical input due to a loss of the visual filter function of the pulvinar. Reproduced with permission from Dr. Ramon Mocellin.
Focal Causes of Visual Hallucinations
Retinal Pathology.
Traction, irritation, injury, or disease of the retina can stimulate retinal photoreceptors, causing brief simple hallucinations in the form of flashes, sparks, or streaks of light. Often both the condition and hallucinations are monocular, and insight is invariably preserved.
Charles Bonnet Syndrome.
In 1769, Charles Bonnet described complex VHs of people, birds, and buildings in his cataract-affected grandfather and later experienced similar phenomena himself.7 The Charles Bonnet syndrome describes a wide variety of VHs associated with visual impairment of any cause—in clear sensorium, with retained insight and without other psychopathology. Typically the visions are colorful images of people, animals, and inanimate objects, occurring especially later in the day, in poor light, or in isolation. Charles Bonnet syndrome has been reported in 12%–65% of visually impaired individuals, particularly in women and with increasing age (mean onset at 74.5 years) and reduced cognitive reserve, with white matter lesions on MR imaging, and with polypharmacy.8,9 Although Charles Bonnet syndrome was initially described in ocular causes of reduced visual input, more recently the term is increasingly used as a catchall denoting complex VHs arising from lesions affecting vision anywhere along the primary visual pathway from the retina onward. The frequency of underlying causes reflects the most prevalent conditions affecting vision, particularly in the elderly: age-related macular degeneration, glaucoma, diabetic retinopathy, and cerebral infarction.8,9
Imaging Features.
A discussion of all causes of Charles Bonnet syndrome is clearly beyond the scope of this article, and many cases will be obvious. A careful review of the globes, usually not the focus of attention in patients undergoing brain imaging, is however useful in potentially alerting the clinician to causes of visual loss as an etiology of complex visual hallucinations. Calcified optic nerve drusen (hyaline calcific deposits) are usually incidental findings; however, they may sometimes be associated with visual field loss or macular degeneration and appear on CT as punctate calcifications at the optic nerve insertion.10 Phthisis bulbi, from prior trauma or infection, may be evident as a small hyperattenuated globe, with a thickened and calcified sclera. Chronic retinal detachment typically appears as subretinal fluid of variable attenuation on CT and signal intensity on MR imaging. Evidence of a prior ocular operation may be evident in the form of scleral buckling or intraocular lens replacement.
Space-Occupying and Vascular Lesions.
Structural disruptions to the visual pathway, for example from neoplastic or vascular lesions, may also lead to complex VHs. In some cases, these are the result of reduced visual input (Charles Bonnet syndrome), whereas in many other instances, the lesions result in VHs without significant loss of vision, supporting the concept of a cortical release of activity from the intact neighboring visual cortex. In historical case series, approximately one-fourth of patients with temporal lobe tumors11 and 15% with occipital tumors12 had VHs, the latter usually simpler in content. Posterior cerebral artery infarction leading to lesions in the occipital cortex or visual thalamus may also lead to VHs, usually restricted to the abnormal visual field.13 In most cases, the hallucinations came days to weeks after the initial infarct and resolved during a period of weeks.
Peduncular Hallucinosis.
These complex and vivid hallucinations arise in the context of lesions in the midbrain pons or thalamus, not just the cerebral peduncles. They can be due to a wide range of pathologic states, including vascular, infectious, neoplastic, and compressive lesions.14,15 These lead to visual hallucinations via disruptions to ascending inputs to the visual pathway, such as inhibitory afferents to the dorsal lateral geniculate nucleus, which then project aberrantly to the visual cortex (Fig 3B).
Imaging Features.
Attention should be paid to the brain stem, in particular the cerebral peduncles, pons, and midbrain, for intrinsic or compressive pathology. Peduncular hallucinosis has been reported following infarcts affecting the cerebral peduncle, as well as compression from lesions such as medulloblastoma and meningioma.15
Posterior Cortical Seizures.
Aberrant electrical activity arising anywhere along the primary or ascending visual pathways leading to focal seizures may result in VHs. Occipital seizures, occurring in approximately 5% of patients with epilepsy,16 are frequently associated with visual manifestations. These are often experienced as simple brief fragmentary stereotyped flashing lights, patterns, or blobs of color or distortions and illusions. Seizures associated with complex VHs suggest involvement of the secondary visual cortex or may arise in association with other symptoms of a peri-ictal psychosis.15
Migraine.
Between 15% and 30% of people with migraines experience auras; of these, 90% are visual.17⇓–19 As with seizures, visual hallucinations and distortions associated with migraine aura are usually simple: The classic aura is of a flickering central zigzag line or crescent progressing peripherally, leaving a central scotoma. Colored patterns and more complex hallucinations may also occur, particularly in rarer causes of migraine, such as familial hemiplegic migraine and migraine coma. Spreading depression of cortical activity may be important in the generation of an aura, with pathologic excitation in visual areas responsible for complex VHs in migraine.15 Notable neuroimaging findings in migraine are well described.20,21
Posterior Reversible Encephalopathy Syndrome.
Posterior reversible encephalopathy syndrome is a radiologic and clinical neurotoxic state secondary to failure of cerebral autoregulation in response to acute changes in blood pressure in patients with eclampsia and posttransplantation states and in a range of other conditions.22 While visual symptoms (including cortical blindness, homonymous hemianopia, blurred vision, and neglect) are relatively common,23 hallucinations are less common, though several cases including complex VHs have been reported.24 Diagnosis relies on strong clinical suspicion and characteristic MR imaging/CT features. Patients may present with severe headache, confusion, visual disturbance, nausea, vomiting, and seizures; recovery occurs relatively quickly following treatment, with resolution of both clinical and radiologic deficits.23 Key neuroimaging features in posterior reversible encephalopathy syndrome are well known (On-line Fig 1).25
Reversible Cerebral Vasoconstriction Syndromes.
Reversible cerebral vasoconstriction syndromes encompass Call-Fleming syndrome (reversible cerebral arterial segmental vasoconstriction), migrainous vasospasm, benign angiopathy of the central nervous system, postpartum angiopathy, and drug-induced arteritis.26,27 These conditions share underlying reversible segmental or multifocal cerebral vasoconstriction and carry the risk of ischemic deficits because of vasoconstriction. Patients may present with sudden-onset posterior thunderclap headache (with or without associated neurologic symptoms) and/or recurrent headaches associated with nausea, vomiting, photophobia, and phonophobia.27,28 Visual hallucinations are a rare manifestation, though again cases have been reported.29 Important neuroimaging features of reversible cerebral vasoconstriction syndromes are widely known.30
Global Causes of Visual Hallucinations
Visual hallucinations arise in a wide range of other neurologic and systemic disorders, due to localized structural disruption from neurofibrillary tangles or synuclein deposition or to widespread neurochemical derangement in neurometabolic disorders, intoxication/withdrawal states, and delirium.
Synucleinopathies with Lewy Body Formation.
Synucleinopathies are a diverse group of related neurodegenerative diseases with a high incidence of VHs characterized by abnormal α-synuclein metabolism, which, in some instances, results in the formation of intracellular inclusions known as Lewy bodies.31,32 The number and distribution of Lewy bodies, particularly in mesial temporal structures, are associated with the frequency of VHs.33 VHs may also relate to synuclein deposition in visual areas, altered ascending input from loss of serotonergic and cholinergic brain stem nuclei, and the use of dopaminergic medications.34 In contrast, synucleinopathies without Lewy bodies, such as multisystem atrophy, have a low incidence of visual hallucinations.35
Lewy Body Dementia.
Lewy body dementia is the second most common form of dementia after Alzheimer disease. Visual hallucinations form part of the core clinical diagnostic criteria for Lewy body dementia and are typically seen early in the course of the disease, before the development of Parkinsonian motor symptoms.36 The incidence of VHs in Lewy body dementia varies between 20% and 75%,31,33,35 and the presence of VH provides an 83% positive predictive value for distinguishing Lewy body dementia from Alzheimer disease.37 VHs in Lewy body dementia typically manifest as prolonged well-formed complex scenes of figures and objects and provoke varied reactions from fear through to indifference.
Imaging Features.
A lack of mesial temporal atrophy in Lewy body dementia is perhaps the most useful finding in distinguishing Lewy body dementia from Alzheimer disease.38⇓–40 A pattern of relatively focused atrophy of the midbrain, hypothalamus, and substantia innominata, with a relative sparing of the hippocampus and temporoparietal cortex, may be seen (On-line Fig 2).
Parkinson Disease/Parkinson Disease Dementia.
Parkinson disease is one of the most common neurodegenerative diseases, seen in 1% of patients older than 60 years of age.41 Patients may present with the classic motor triad of tremor at rest, rigidity, and hypokinesia, as well as a range of nonmotor symptoms. VHs are common and occur in 25%–50% of patients with Parkinson disease42 and are similar in content to those of Lewy body dementia, ranging from people or animals to complex, formed, and animated scenes.
Imaging Features.
In most instances, imaging plays a supportive role in the diagnosis of Parkinson disease,39 which is usually established clinically. Loss of normal susceptibility-induced signal drop-out in the substantia nigra pars compacta on T2*-weighted images is potentially the most useful feature, but this has been difficult to demonstrate reliably.43 Other features include mild T1 signal hyperintensity of the reticular parts of the substantia nigra and red nuclei and dotlike areas of hyperintensity in the compact part of the substantia nigra; however, the clinical utility of such findings is limited because they are subtle and are only reported late in the disease.44,45
Alzheimer Disease/Posterior Cortical Atrophy.
VHs may also be seen in Alzheimer disease, particularly in patients with advanced disease and when combined with confusion and loss of visual acuity.37,46 VHs in Alzheimer disease may result from Alzheimer plaques and tangles in the visual-association cortices and have been associated with periventricular white matter lesions and occipital atrophy.47,48
VHs are seen in approximately 25% of patients diagnosed with the posterior cortical atrophy variant of Alzheimer disease, in which cortical loss is localized, particularly to the occipital and parietal lobes, leading to visual agnosia and apraxia.49 Those patients with posterior cortical atrophy and complex VHs have disproportionate involvement of the midbrain, thalamus, and primary visual cortex, and interplay between these regions may be responsible.49
Imaging Features.
Cortical atrophy tends to occur within the mesial temporal structures, with widening of the parahippocampal fissures. SPECT and FDG-PET examinations demonstrate reduced bitemporoparietal uptake, reflecting reduced cerebral blood flow. The presence of parietal-predominant volume loss is suggestive of the posterior cortical atrophy variant.50 MR imaging demonstrates gray matter atrophy involving the occipital, parietal, and posterior temporal lobes, often more pronounced on the right side (On-line Fig 3).51 In patients with visual hallucinations, additional regions involved include the primary visual cortex, thalamus, basal nuclei, midbrain, basal forebrain, and posterior frontal and medial temporal lobes.49
Frontotemporal Lobar Degeneration.
Frontotemporal lobar degeneration covers a spectrum of genetically and neuropathologically heterogeneous disorders, including behavioral variant frontotemporal dementia, semantic dementia, and progressive nonfluent aphasia.52 Frontotemporal lobar degeneration leads primarily to personality and behavioral changes and language disturbance but also to psychotic symptoms in 10%–30% of cases53,54 and visual hallucinations in up to 14%.54 Psychotic symptoms are more prevalent in carriers of the C9orf72 mutation, whose thalamus and cerebellum are more frequently affected.55 VHs are more common in the right than in the left temporal variant frontotemporal lobar degeneration,56 often associated with delusions.57
Imaging Features.
While atrophy in the anterior temporal and medial frontal lobes is characteristic of frontotemporal lobar degeneration (On-line Fig 4),58 specific imaging findings can reflect underlying subtypes. Bilaterally symmetric or right frontal atrophy is seen in the behavioral subtype. In the semantic dementia subtype, there is anterior temporal–predominant atrophy. Left dominant atrophy is seen if speech apraxia predominates, with right dominant atrophy if prosopagnosia predominates.40 Frontostriatal dysfunction also varies among these different subtypes, with the behavioral variant having the greatest involvement: Caudate heads are relatively reduced in size in these patients compared with those with the language variant of frontotemporal lobar degeneration.59
Creutzfeldt-Jakob Disease.
In Creutzfeldt-Jakob disease, a rare, rapidly progressive neurodegenerative condition caused by prion infection, VHs may accompany the typical rapid cognitive decline, anxiety, personality change, myoclonic jerks, and ataxia.60 Visual effects may include color changes, field defects, visual agnosia, and distortions progressing to frank hallucinations,61 seen particularly in the Heidenhain variant of CJD and associated with periodic electroencephalography complexes over the occipital region.62
Imaging Features.
Sporadic CJD classically results in cortical diffusion restriction as the earliest imaging manifestation (On-line Fig 5), which may be bilateral or unilateral, symmetric or asymmetric. Bilateral areas of increased signal intensity predominantly affecting the caudate nuclei and the putamina should also suggest the diagnosis of CJD.63 In variant CJD, FLAIR/T2 hyperintensity may be demonstrated in the pulvinar nuclei bilaterally (pulvinar sign) and both the dorsomedial thalamus and pulvinar (hockey stick sign).64,65
Intoxication, Withdrawal, and Delirium.
Delirium tremens, seen in severe alcohol withdrawal, is associated with frightening VHs, tremor, autonomic disturbance, and agitation.15 Similar withdrawal states may follow the sudden cessation of benzodiazepines or barbiturates, suggesting a shared role of altered γ-aminobutyric acid signaling.66 Drugs such as lysergic acid diethylamide and mescaline have hallucinogenic properties correlated with their serotonergic activity and lead to colored patterns, distortions, and illusions that progress to include complex scenes of animals and people. These are often vivid and associated with heightened sensory arousal, with preserved insight and without paranoia or delusional interpretation. Cocaine and amphetamines in contrast, which act to increase synaptic dopamine transmission, tend to produce VHs with heightened paranoia and agitation.66
Delirium is a syndrome of disturbed consciousness and impaired attention associated with a raft of metabolic, infectious, toxic, and intracranial causes.67 Hallucinations are often a prominent part of this syndrome and are typically visual, with vivid, complex, and often frightening scenes of people and animals that may be accompanied by paranoia and fleeting delusions.66
Imaging Features.
In most cases of intoxication, withdrawal, and delirium, imaging is performed to rule out underlying structural pathology. MR imaging in Wernicke-Korsakoff syndrome, induced by thiamine deficiency in starvation and alcoholism, demonstrates T2 hyperintensity in the mammillary bodies, thalami, periaqueductal gray tectal plate, and dorsal medulla, with possible associated contrast enhancement.68
Psychiatric and Other Causes of Visual Hallucinations
Once focal and global brain pathology has been excluded with MR imaging and other investigations, psychiatric causes including major affective and psychotic disorders should be considered. Brain MR imaging findings are usually normal.69 In schizophrenia, VHs are around half as common as auditory hallucinations; when experienced by people with schizophrenia, VHs are also usually accompanied by auditory hallucinations.70,71 In one sample, visual hallucinations were present in 16% of subjects and were related to the severity of illness.72 Visual hallucinations are also common in states of reduced consciousness, such as entering and awakening from sleep, particularly in the presence of sleep disorders,73 and may be induced by prolonged visual deprivation, a syndrome akin to that described in Charles Bonnet syndrome.
Conclusions and Imaging Recommendations
Considering the range of focal and global pathology that can result in VHs, a sensible approach to imaging is needed (On-line Table). Often the type or features of VHs being experienced are not indicated on imaging requests, so highly targeted protocols are unreliable. Instead, we recommend a relatively generic approach able to adequately image the entire optic pathway and identify, if not necessarily fully characterize, all likely pathologies. The key sequences are high-resolution T1 and T2/FLAIR, preferably isotropic volumetric imaging, susceptibility-weighted imaging, and diffusion-weighted imaging. Time permitting, additional catchall sequences may be added (eg, MR perfusion, double inversion recovery, MRA).
A systematic approach to the review of these sequences with regard to direct and ascending visual pathways looking first for focal and patterns of global pathologies outlined above will ensure detection of the most important pathology underlying the presentation of visual hallucinations (Table 3).
Reported incidence of visual hallucinations in various conditions
Footnotes
Disclosures: Toby T. Winton-Brown—UNRELATED: Grants/Grants Pending: Wellcome Trust, UK (Research Training Fellowship, WT087779MA). Dennis Velakoulis—UNRELATED: Royalties: Neuropsychiatry Unit Cognitive Assessment Tool; Stock/Stock Options: Prana Biotechnology Ltd, a company with research into neurodegenerative disorders. Frank Gaillard—UNRELATED: Employment: Radiopaedia.org (Founder, Editor, and CEO).
Indicates open access to non-subscribers at www.ajnr.org
References
- © 2016 by American Journal of Neuroradiology