Abstract
BACKGROUND AND PURPOSE: Cervical transforaminal epidural steroid injections are commonly performed for temporary pain relief or diagnostic presurgical planning in patients with cervical radiculopathy. Intravascular injection of steroids during the procedure can potentially result in cord infarct, stroke, and even death. CT-fluoroscopy allows excellent anatomic resolution and precise needle positioning. This study sought to determine the safest needle tip position during CT-guided cervical transforaminal epidural steroid injection as determined by the incidence of intravascular injection.
MATERIALS AND METHODS: We retrospectively evaluated procedural imaging for consecutive single-site CT-fluoroscopic cervical transforaminal epidural steroid injection performed during a 13-month period. Intravascular injections were identified and classified by volume, procedure phase, vessel type, and needle tip position relative to the targeted neural foramen. ANOVA, Wilcoxon, or Pearson χ2 testing was used to assess differences among groups as appropriate.
RESULTS: Intravascular injections occurred in 49/201 (24%) procedures. Of the intravascular injections, 13/49 (27%) were large, 10/49 (20%) were small, and 26/49 (53%) were trace volume. Sixteen of 49 (33%) intravascular injections occurred with a trial contrast dose; 27/49 (55%), with a steroid/analgesic cocktail; and 6/49 (12%), with both. Twenty-seven of 49 (55%) intravascular injections were likely venous, 22/49 (45%) were indeterminate, and none were likely arterial. The intravascular injection rate was significantly lower (P < .001) for the extraforaminal needle position (8/82, 10%) compared with junctional (27/88, 31%) and foraminal (14/31, 45%) needle tip positions.
CONCLUSIONS: An extraforaminal needle position for CT-guided cervical transforaminal epidural steroid injection decreases the risk of intravascular injection and therefore may be safer than other needle tip positions.
ABBREVIATION:
- TFESI
- transforaminal epidural steroid injection
Cervical radiculopathy is a common medical condition with a reported annual incidence of 0.8/1000 persons.1 Cervical transforaminal epidural steroid injections (TFESIs) are commonly performed in patients with cervical radiculopathy to provide targeted diagnostic information to referring surgeons or to provide short-term pain relief. CT-fluoroscopy offers excellent anatomic resolution and allows very precise needle positioning, making it the preferred technique for many proceduralists.2,3 Posterior circulation stroke and cord infarct are rare but potentially devastating complications of cervical TFESIs.4⇓⇓⇓⇓–9 Although some debate remains, these complications are most commonly attributed to accidental intravascular injection of steroid.4
The intravascular injection rate for CT-guided cervical TFESIs has previously been estimated at 1%–26%,3,10,11 while the corresponding rate for conventional fluoroscopic guidance has been published at 17%–32.8%.12⇓⇓–15 Despite the known risks of the procedure and the high anatomic resolution of CT, the role of needle position in intravascular injection has not been previously evaluated, to our knowledge.
The purpose of this study was to determine the safest needle tip depth relative to the targeted neural foramen as determined by the incidence of intravascular injection. We also characterized intravascular injections by volume, phase of the procedure (contrast injection versus steroid/analgesic cocktail), and likely vessel type injected.
Materials and Methods
Subjects
Local institutional review board approval was obtained for this retrospective review of clinical and imaging data. This study was compliant with the Health Insurance Portability and Accountability Act.
We retrospectively searched our radiology information system for all consecutive unilateral, single-level CT-guided cervical TFESIs performed for upper extremity radiculopathy by the neuroradiology division at our main academic campus during a 13-month period (February 2014 to February 2015). C3-level injections performed for radiculopathy were included, but C3-level procedures performed for occipital neuralgia were excluded.
Procedure Technique
All procedures were performed by 1 of 3 attending neuroradiologists (G.M.L., W.E.R., and V.A.) with Certificates of Added Qualification in neuroradiology and having 4, 24, and 8 years of experience, respectively, performing image-guided spine procedures. The injections were performed by using a technique similar to that previously published3 with additional details as follows: All procedures were performed on a single LightSpeed Plus 4-detector row CT scanner (GE Healthcare, Milwaukee, Wisconsin). Scout imaging was acquired through the targeted level by using the following parameters: rotation time, 0.8 seconds; speed, 3.75 mm/rotation; pitch, 0.75:1; section thickness, 2.5 mm; 120 kV with variable milliamperes; and noise index, 4.69. Intermittent CT-fluoroscopy was performed with SmartView (GE Healthcare) activated by a foot pedal and creating 3 consecutive, 2.5 mm-thick, axial sections per scan by using 120 kV with variable milliamperes. All CT-fluoroscopy imaging acquired during the procedure was automatically archived to our PACS.
A 25-ga, 3.5-inch Quincke spinal needle (BD Medical, Franklin Lakes, New Jersey) was advanced toward the posterior margin of the targeted neural foramen. The posterior margin of the foramen was targeted because it has been suggested that the posterior aspect of the foramen is less vascular and may carry less risk of intravascular injection.2 Furthermore, targeting the posterior neural foramen aids in avoiding injury to the vertebral artery.
After attachment of flexible microbore tubing, a trial dose of 0.3 mL of iohexol contrast agent (Omnipaque, 180 mg/mL; GE Healthcare, Piscataway, New Jersey) was injected and was immediately followed by CT-fluoroscopy to evaluate for intravascular contrast. (We use the term “trial dose” for the contrast-only injection to distinguish from “test dose,” a term historically used to refer to the injection of analgesic before steroid injection.) If intravascular injection was identified with the trial dose, the needle was withdrawn a few millimeters and a repeat injection of 0.3 mL of iohexol was performed with repeat CT-fluoroscopic imaging. These steps were repeated until there was no evidence of intravascular injection.
A cocktail of 1.2 mL of 2.5 or 5 mg/mL bupivacaine analgesic, 8-mg of preservative-free dexamethasone sodium phosphate steroid (10 mg/mL), and 0.3 mL of iohexol was then injected under additional CT-fluoroscopic guidance. We use dexamethasone as our glucocorticoid for cervical TFESIs because its nonparticulate nature may reduce the risk of stroke or cord infarct if the steroid is accidentally injected intravascularly.4,5 No case of cord infarct or stroke has been reported with nonparticulate steroids to date.16
The patient was monitored for 15 minutes after the procedure for minor complications (such as vasovagal response or increasing postprocedure pain) and major complications (such as cardiovascular or neurologic compromise).
Image Evaluation
All studies were retrospectively evaluated by 2 of the proceduralists (G.M.L. and V.A.) blinded to operator and patient identity. The 2 reviewers initially evaluated and characterized all imaging separately. In cases of disagreement, the 2 re-evaluated the relevant imaging together and reached a consensus on all findings and characterizations.
Intravascular Injection Definition
Intravascular injection was considered present if 1 of 2 contrast appearances was identified on CT-fluoroscopy, similar to previously described criteria11:
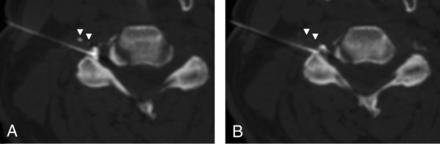
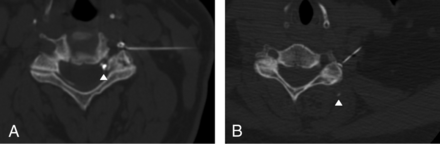
1) Contrast appeared as ≥1 round or curvilinear discrete foci separate from the needle tip and its surrounding epidural contrast collection (Fig 1). In this situation, the initial intravascular contrast was sometimes confirmed by partial or complete dissipation of the discrete foci of contrast on immediate repeat CT-fluoroscopic imaging, though this additional confirmation was not required for classification as intravascular contrast.
2) Either far less than the expected amount of injected contrast, or no contrast at all, accumulated adjacent to the needle or elsewhere on the imaging (Fig 2). In this context, we inferred that a vessel had rapidly carried the contrast out of the plane in the very short time between injection and imaging. Complete absence of contrast was considered a completely intravascular injection.
Mixed intravascular and epidural contrast injection. A, Intravascular injection appears as discrete foci of contrast (arrowheads) away from the needle tip and adjacent main, epidural contrast collection. B, The intravascular contrast almost completely disappears (arrowheads) on immediate repeat CT-fluoroscopic imaging. This rapid resolution of contrast confirmed but was not required for identification of intravascular contrast. The needle tip position is junctional on these images.
Intravascular injection identified by a less-than-expected accumulation of epidural contrast. A, Needle position before the contrast trial dose. B, Only a very small amount of contrast, considerably less than the injected volume of 0.3 mL of iohexol, is seen on immediate postinjection imaging. The missing contrast is inferred to be intravascular and has been circulated out of the imaged field. (A trace amount of intravascular contrast is also noted within the right aspect of the spinal canal.) C, After the needle is withdrawn several millimeters, a repeat contrast trial injection shows the expected volume of injected contrast accumulating in the epidural space. No additional intravascular contrast was identified with the steroid/analgesic injection (not shown), making this a trial dose intravascular injection.
Contrast appearing as a continuous curvilinear collection extending away from the needle tip between paraspinal muscles was interpreted as contrast extending within the fascia planes and did not represent intravascular contrast.
Intravascular Injection Volume
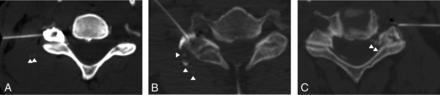
Intravascular injections were characterized by volume into 1 of 3 categories (Fig 3):
1) Trace (Fig 3A): Intravascular contrast appears as 1–2 foci, each appearing as a punctate or curvilinear focus and measuring ≤2 mm in diameter.
2) Small (Fig 3B): Intravascular contrast volume is too small to create a clear perceptible decrease in the expected volume of accumulating epidural contrast. However, the visualized intravascular contrast component either has a smallest transaxial dimension measuring ≥3 mm or appears as ≥3 separate foci.
3) Large (Fig 3C): The amount of accumulating epidural contrast is clearly smaller than the injected amount. In this case, the nonvisualized contrast was interpreted as already carried out of the imaged FOV by the vascular system.
Intravascular contrast injection classified by volume. A, Trace volume of intravascular injection appears as 1–2 subtle foci (arrowheads), each ≤2 mm. The image is windowed to accentuate the intravascular contrast; the initial appearance on default window settings is even subtler and was not identified at the time of the procedure. B, A small volume of intravascular injection appears either as ≥3 foci (arrowheads), at least 1 focus of ≥3 mm (central arrowhead), or both (as in this case). C, A large volume of intravascular contrast injection. Less than the expected volume of injected iohexol is seen on the imaging because most of the intravascular contrast has already been circulated out of the FOV. Some intravascular contrast is present within the venous plexus both adjacent to the needle tip and more medially (arrowheads).
Intravascular Injected Material
The intravascular injection was classified as occurring with the trial contrast dose, the steroid/analgesic injectate, or both.
A trial dose intravascular injection was considered present if 3 criteria were satisfied (Fig 2):
1) Intravascular injection was present on the first imaging acquired immediately after the initial trial dose.
2) Needle withdrawal and repeat trial dose were mentioned in the report of the procedure or could be identified on the imaging.
3) Intravascular injection was not identified, even in retrospect, on the ensuing injection of steroid/analgesic.
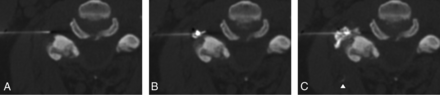
Steroid/analgesic intravascular injection was considered present if 2 criteria were satisfied (Fig 4):
1) Intravascular injection was not seen, even in retrospective analysis, during the contrast trial dose.
2) Intravascular contrast was identified in retrospect during the ensuing injection of steroid/analgesic.
Intravascular injection seen only on steroid/analgesic injection. A, Needle position preinjection. B, Contrast trial injection shows no intravascular injection. C, Subsequent injection of the steroid/analgesic cocktail shows a small intravascular injection (arrowhead) within an indeterminate paraspinal vessel. The needle was unchanged in position between trial injection and steroid/analgesic cocktail injection.
An intravascular injection with both a trial dose and steroid/analgesic components was considered present if 3 criteria were satisfied (Fig 5):
1) Intravascular contrast was present in retrospect on trial-dose imaging.
2) Needle withdrawal and the repeat trial dose were not mentioned in the report of the procedure and could not be identified on the imaging.
3) Additional intravascular contrast was identified in retrospect on the ensuing injection of steroid/analgesic.
Intravascular injection on both trial injection and steroid/analgesic cocktail injection. A, The trace intravascular injection (arrowhead) is subtle but present on the contrast trial dose; the proceduralist did not appreciate it at the time of the procedure. B, More obvious intravascular injection (arrowhead) is evident on the ensuing steroid/analgesic cocktail injection.
In this final situation, the 2 components of intravascular injection are intimately related and closely dependent. Thus, we consider this situation to represent a single intravascular injection with components in 2 phases: one trial dose and the other steroid/analgesic injectate.
Vessel Characterization
Intravascular injections were characterized as likely venous, likely arterial, or indeterminate, as shown in Fig 6. In likely venous, contrast accumulates away from the needle tip within the foraminal venous plexus. In indeterminate, discrete contrast accumulates away from the needle tip within or between the paraspinal muscles, in the expected region of branches of the anterior cervical artery and small draining veins. In likely arterial, contrast clearly extends along the anatomic course of the vertebral artery, ascending cervical artery, radiculomedullary artery, or anterior spinal artery.
Classification of intravascular injection by vessel type. A, Venous injection, with contrast within extracanalicular and intracanalicular (arrowhead) components of the venous plexus. B, Indeterminate vessel injection, with contrast accumulating within a small paraspinal vessel (arrowhead). This could represent either a branch of the ascending cervical artery or a small vein. No likely arterial injections were identified.
Needle Depth
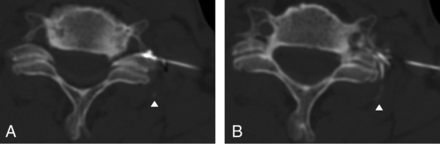
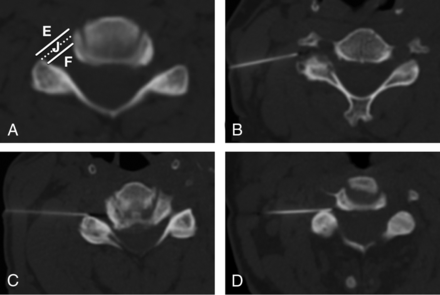
Needle tip position at the time of injection was classified as extraforaminal, junctional, or foraminal by using a modified version of a previously published scheme (Fig 7).10,17 The lateral junction of the targeted neural foramen was defined by a line connecting the anterolateral margin of the vertebral body with the lateral margin of the facet joint. For the low cervical neural foramina, where the transverse process is a lateral continuation of the facet joint, the transverse process was not considered a part of the facet joint. A needle tip within 2 mm of this line segment was characterized as within the junctional zone. A needle tip lateral to the junctional zone was classified as extraforaminal; a needle tip medial to the junctional zone was classified as foraminal.
Needle depth classification. A, The lateral junction of the neural foramen (dotted line) is defined by a line segment connecting the anterolateral margin of the vertebral body with the most lateral margin of the facet joint. A needle tip in a zone within 2 mm medial or lateral to this segment (solid lines) is classified as within the junctional zone (J). A needle tip >2 mm lateral is classified as within the extraforaminal zone (E), and a needle tip >2 mm medial is classified as within the foraminal zone (F). Examples of extraforaminal (B), junctional (C), and foraminal (D) needle tip positions.
Statistical Testing
Pearson χ2 testing was used to assess differences in vascular injections based on needle position. Differences in vascular injections were assessed on the basis of age, sex, and prior surgical history by using ANOVA, Wilcoxon, and Pearson χ2 testing, respectively. If appropriate, post hoc multiple comparison testing was performed with correction (the Tukey method and the Steel-Dwass procedure for parametric and nonparametric tests, respectively). Statistical testing was performed by using JMP 11 (SAS Institute, Cary, North Carolina).
Results
Patient Population
A total of 175 patients underwent 201 consecutive unilateral, single-level procedures, with most (87%) undergoing only a single cervical injection during the 13-month study period. The remaining 13% of patients underwent ≥2 unilateral, single-level injections on different days. The mean patient age was 53 years (range, 25–87 years). Fifty-three percent of procedures were performed on men; 47% were performed on women. The most frequently injected levels were C6 and C7, with a roughly equal split in laterality. There was no significant difference in age, sex, or history of prior cervical surgery among groups based on needle depth or vascular injection (P > .05).
Intravascular Injection Incidence, Volume, and Material
Intravascular injections occurred in 49/201 (24%) procedures. Of these procedures, 16/49 (33%) had intravascular injection only with the trial dose; 27/49 (55%), only with the steroid/analgesic injection; and 6/49 (12%), with both the trial dose and the steroid/analgesic injectate. Regarding volume, 13/49 (27%) were large, 10/49 (20%) were small, and 26/49 (53%) were trace volume.
Injected Material and Volume of Intravascular Injection
Of the 16 intravascular injections occurring during the trial dose only, 10/16 (63%) were large volume, 3/16 (19%) were small volume, and 3/16 (19%) were trace volume intravascular injections. Of the 27 intravascular injections occurring with steroid/analgesic injectate only, 2/27 (7%) were large volume, 3/27 (11%) were small volume, and 22/27 (81%) were trace volume intravascular injections. All 6 intravascular injections with both trial dose and steroid/analgesic components had the same size intravascular injection in the 2 components: One of 6 (17%) injections was large volume; 4/6 (67%), small volume; and 1/6 (17%), trace volume.
Injected Vessel
Regarding injected vessels, 27/49 (55%) were likely venous, 22/49 (45%) were indeterminate, and none were likely arterial.
Type of Vessel Injected and Size of Intravascular Injection
Of the 13 large-volume intravascular injections, 11/13 (85%) were likely venous and the remaining 2/13 (15%) were associated with an indeterminate vessel type. Of the 10 small-volume intravascular injections, 8/10 (80%) were likely venous and the remaining 2/10 (20%) had an indeterminate vessel type. Of the 26 trace-volume intravascular injections, 8/26 (31%) were likely venous and the remaining 18/26 (69%) had an indeterminate vessel type.
Injected Material and Type of Vessel Injected
Of the 16 intravascular injections occurring on trial dose only, 13/16 (81%) were likely venous, and in the remaining 3/16 (19%), the injected vessel type was indeterminate. Of the 27 intravascular injections occurring with the steroid/analgesic cocktail only, 10/27 (37%) were likely venous and 17/27 (63%) were indeterminate for vessel type. For the 6 intravascular injections with both trial dose and steroid/analgesic components, 4/6 (67%) were likely venous and 2/6 (33%) were indeterminate for vessel type.
Needle Depth
Needle depth at the time of injection was more commonly extraforaminal (82/201, 41%) or junctional (88/201, 44%) and less often foraminal (31/201, 15%).
The intravascular injection rate was significantly lower (P < .001) for extraforaminal needle position (8/82, 10%) compared with junctional (27/88, 31%) and foraminal (14/31, 45%) needle tip positions (pair-wise comparisons: extraforaminal versus junctional, P < .001; extraforaminal versus foraminal, P < .001; junctional versus foraminal, P = .145).
Complications
There were no minor or major intraprocedural or immediate postprocedural complications.
Discussion
Intravascular injection during CT-fluoroscopic cervical TFESI is common, occurring in 24% of our 201 cases. Of these, most (53%) were trace volume intravascular injections, and most resulted in intravascular injection of the steroid and analgesic. Extraforaminal needle tip position strongly correlated with a lower incidence of intravascular injection (P < .001). Despite many intravascular injections of nonparticulate steroid, there were no complications.
We have shown that the extraforaminal needle tip position may be relatively safe because it reduces the risk for intravascular injection. Minimizing rates of intravascular injection is critical because accidental embolization of steroid and analgesic can potentially lead to rare but catastrophic complications, such as cord infarct, stroke, or even death.4⇓⇓⇓⇓–9
There has been considerable disagreement regarding the ideal needle position within the targeted neural foramen for CT-guided cervical TFESIs. Some assume that a relatively deep position, with the needle tip within the outer neural foramen and immediately adjacent to the targeted nerve root, is required for proper analgesic and steroid efficacy,3,10,18 while others advocate a more cautious, extraforaminal needle tip position to minimize the risk of complications.8,19,20 Junctional and foraminal needle tip positions have been previously shown to have higher rates of foraminal contrast flow compared with the extraforaminal needle position,10 though contrast dispersal pattern was shown not to correlate with pain relief in 1 study.21
To our knowledge, we are the first to observe, on CT-guided TFESI, contrast appearing within vessels during steroid/analgesic cocktail injection, a finding we believe depicts intravascular injection of steroid and analgesic. We were able to evaluate intravascular injection of the steroid/analgesic cocktail because we routinely mix iodinated contrast with our cocktail, a procedural technique detail not practiced at many other institutions. In previous descriptions of CT-guided3,10,11 and conventional fluoroscopic-guided12⇓⇓–15 injections, the intravascular contrast was always identified on the trial dose and the needle was appropriately readjusted before injecting the steroid/analgesic cocktail. Many of our intravascular injections were trace volume, had a very subtle appearance, were not noted at the time of the procedure, and were identified only with meticulous retrospective evaluation of the procedural imaging. It is possible that intravascular injections of steroid and analgesic occurred in these prior studies but were not detected. Kranz et al11 reported an overall rate of intravascular injection during CT-fluoroscopic cervical TFESI similar to our own (26% versus our 24%), but with all occurrences identified only on the trial contrast injection. We may have a relatively high rate of steroid/analgesic intravascular injection because we do not use the “double tap” technique of Kranz et al to evaluate intravascular contrast washout with the trial contrast dose injection. A future study directly comparing procedures performed with and without the double tap technique is warranted to prove the effectiveness of the double tap technique.
Intravascular injection occurred more often with the steroid/analgesic cocktail than with the trial dose. The reason is not completely clear, but we speculate that the larger overall volume of the steroid/analgesic cocktail (>2 mL of combined analgesic, steroid, and contrast) compared with the trial dose (0.3 mL of contrast alone) leads to slightly greater conspicuity of punctate intravascular injections, which constituted most steroid/analgesic intravascular injections.
Although we identified many likely intravascular injections of contrast/steroid injectate (27 procedures with intravascular injection during the steroid/analgesic phase alone and 6 procedures during both trial dose and contrast/steroid injection), we had no complications in the course of 201 procedures. Other authors have similarly reported no complications in 30 injections on 30 patients18 and 90 injections on 63 patients10 (though comparison may be imperfect because those studies did not mix contrast with their steroid injection). Major complications seem to occur at a <0.1% incidence.17 This safety record indicates that CT-fluoroscopic-guided cervical TFESIs are safe when performed by well-trained proceduralists who are experienced in spine procedures. In particular, spine procedures using nonparticulate steroid such as dexamethasone appear to carry a low risk of stroke or spinal cord infarct from embolized steroid.4,22 Earlier reports of complications from intravascular injection of steroids have often used triamcinolone or methylprednisolone,4,6⇓–8,16 both of which have large aggregate particles. In 1 study performed on pigs, 4/4 injected with methylprednisolone into their vertebral arteries experienced catastrophic strokes and cord infarcts, whereas 0/7 injected with prednisolone or dexamethasone had any complications.23 Regardless of steroid used, the proceduralist must always be mindful of the risk of accidentally directly injuring the vertebral artery with the procedure needle.
Limitations of our study include those inherent in a retrospective design and a single-hospital experience. Lack of relatively long-term (1–2 month) pain relief results also prevents us from recommending an extraforaminal needle position as the ideal position for cervical TFESIs. A future study with appropriate statistical power evaluating long-term pain relief, needle tip position, and intravascular injection incidence is warranted to determine the ideal needle tip position that minimizes intravascular injection while preserving patient pain relief.
Conclusions
An extraforaminal needle position for CT-guided cervical TFESI decreases the risk of intravascular injection and therefore may be safer than other needle tip positions.
Footnotes
Paper previously presented in part at: American Society of Neuroradiology Annual Meeting and the Foundation of the ASNR Symposium, May 25–30, 2015; Chicago, Illinois.
REFERENCES
- Received August 10, 2015.
- Accepted after revision September 14, 2015.
- © 2016 by American Journal of Neuroradiology