Our study commenced in the last quarter of 2013, concluded in May 2014, and was presented at the Symposium Neuroradiologicum held in Istanbul on September 7–12, 2014. As a result of multipdisciplinary authorship and distant collaborations, the manuscript was finally submitted to the American Journal of Neuroradiology in December 2014. At the initial period of our study, there were 2 articles in the literature concluding that hyperintense dentate nuclei (HDN) on T1-weighted MR imaging were secondary to rapidly progressive MS and radiation therapy (RT) effect.1,2 We, therefore, investigated this entity in a large cohort of irradiated individuals and found no apparent associations between HDN and RT. During the data gathering and after the submission of our manuscript, several studies were published on this topic, mainly arguing the possible association of HDN and repeated performance of gadolinium-enhanced MR imaging.3,4 McDonald et al5 were the first to show actual gadolinium deposition in the human brain by using inductively coupled plasma mass spectrometry in postmortem subjects. Although linear gadolinium-based contrast agents were reported to be associated with HDN, the exact mechanism and clinical ramifications remain unclear.6⇓–8
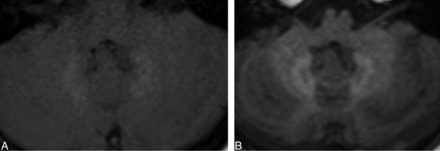
T1-weighted FLAIR is a recently described MR imaging sequence.9 In brain imaging, it is mainly used to better differentiate gray and white matter, owing to its improved contrast difference. In our institution, it is not a part of the standard brain MR imaging protocol and is mainly performed for epilepsy. Because our cohort was obtained from those with a history of RT for underlying tumoral lesions, only a handful of the subjects were imaged with the T1 FLAIR sequence. As far as the authors' qualitative assessment, differentiating HDN from normal dentate nuclei (NDN) on axial T1 FLAIR images was not a matter of debate in any of subjects included in our study (Fig 1). On sagittal T1 FLAIR images, given the enhanced brightness of white matter, qualitative differentiation of faint HDN and NDN could be doubtful, particularly if the reader is not familiar with appearance of HDN. Nevertheless, without specific research on this topic, we do not speculate on the superiority of one technique (T1 FSE, FLAIR, MPRAGE, and so forth) over another in differentiating faint HDN from NDN. From our study cohort, we randomly looked into 7 subjects with NDN who underwent at least 6 contrast-enhanced MR imaging examinations by using linear gadolinium agents (Table). Not a single case was imaged with T1 FLAIR, practically excluding the possibility of HDN being misinterpreted as NDN.
A 16-year-old girl who underwent periodic MR imaging after gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, New Jersey) administration for operated pilocytic astrocytoma of the optic nerve during 14 years. HDN was clearly visible on both sequences (A, FSE T1WI; TE, 526 ms; TE, 12 ms. B, T1 FLAIR; TR, 1200 ms; TE, 2.46 ms; inversion recovery, 600).
Number of contrast-enhanced MRIs and amount of gadolinium administration per scana
The mechanism of gadolinium retention in the dentate nuclei is unknown, and individual factors contributing to the normal appearance of the dentate nuclei in some patients, despite the large amount of gadolinium administered, remain unclear.
Acknowledgments
We thank Kanda et al for their interest in our study and their contribution to the knowledge of gadolinium safety.
References
- © 2016 by American Journal of Neuroradiology