Abstract
BACKGROUND AND PURPOSE: Medial temporal lobe abnormalities on DWI and functional imaging are occasionally observed in patients with transient global amnesia. We used CTP to study these patients during or briefly after resolution of their amnesic syndrome.
MATERIALS AND METHODS: From 2002 onward, patients satisfying clinical criteria for transient global amnesia who underwent CTP were included. Patients with additional clinical features suggesting transient ischemic attack or stroke and those with an ischemic lesion on subsequent DWI were excluded. If deemed necessary by the clinician, DWI was performed within 10 days.
RESULTS: Thirty patients with transient global amnesia underwent CTP at a median latency of 5.9 hours (interquartile range, 4.3–9.7 hours) after symptom onset. All findings, except for those in 1 patient, were normal, including those in the 14 patients with well-imaged hippocampi. In the patient with abnormal findings, CTP and PWI showed hypoperfusion in both lentiform nuclei extending into the insulae, with normalization on the repeat CTP 6 days later. In 10 patients, DWI was performed at a median latency of 2 days (interquartile range, 0–9 days). Of these, 2 showed punctate hippocampal lesions, often seen in transient global amnesia. In 2 patients excluded because of mildly atypical transient global amnesia and ischemic lesions on subsequent DWI, acute CTP findings were also normal.
CONCLUSIONS: Patients with transient global amnesia had normal CTP findings in the acute phase with the exception of 1 patient with transient hypoperfusion in both basal ganglia. If imaging is performed for typical and atypical transient global amnesia, DWI should be the preferred method.
ABBREVIATION:
- TGA
- transient global amnesia
Transient global amnesia (TGA) is characterized by the sudden onset of antero- and retrograde amnesia that is often triggered by an acute emotional or physical event and spontaneously resolves within 24 hours. Repetitive questioning and anxiety are often present, but other focal neurologic disturbances are usually absent.1,2
Functional brain imaging with SPECT and PET shows local disturbances in regional blood flow (usually hypoperfusion, rarely hyperperfusion) and in oxygen/glucose metabolism, most frequently in 1 or both medial temporal lobes.3⇓–5 Punctate signal hyperintensities appearing in 1 or both hippocampi on DWI were first described by Matsui et al6 in a classic TGA case in 2002 and then more systematically by Sedlaczek et al.7 Although the specific sites of impairment have been identified, the underlying etiology of TGA remains elusive.2
Images in a case series of patients with TGA who underwent PWI within 24 hours of symptom onset showed unilateral perfusion abnormalities in 4 of the 28 patients (1 in the anterior, 1 in the posterior, and 2 in the middle cerebral artery territory).8 However, the authors did not specify whether structures relevant for memory were involved.
CTP is widely used for the early diagnosis of acute ischemic stroke and TIA, though its true value remains to be established by appropriate research.9 To our knowledge, results using CTP for acute TGA diagnosis have not been published. We, therefore, aimed to determine the frequency and localization of perfusion abnormalities in patients with acute TGA by using CTP to investigate its potential diagnostic value and to better elucidate the pathogenesis of this disorder.
Materials and Methods
We included and retrospectively analyzed those patients admitted to the emergency department of the University Hospital of Lausanne from June 2002 to April 2014 who had a final diagnosis of TGA and underwent CTP within 24 hours of admission. CTP was part of the standard diagnostic protocol of the hospital to detect cerebral ischemia from June 2002 until April 2006 and has been performed at the discretion of the treating physician since April 2006. All patients underwent neurologic evaluation, and the diagnosis of TGA was made if the criteria of Hodges and Warlow10 were met. Additional examinations such as electroencephalography and DWI were performed as considered necessary by the treating physician. Exclusion criteria were poor-quality CTP and a proved ischemic lesion on DWI after an initial TGA diagnosis.
We collected the following parameters: demographics (age, sex); cerebrovascular risk factors, including hypertension, hyperlipidemia, diabetes mellitus, smoking history, atrial fibrillation, previous cerebrovascular event (stroke or transient ischemic attack) or TGA; high-risk alcohol consumption defined as exceeding 24 g/day; and the presence of a triggering event (emotional or physical). The duration of amnesia was determined by serial bedside testing of the capability to retain at least 4 words of a standardized 5-word list.11,12 Formal neuropsychological testing was performed, depending on the treating physician's judgment.
CTP was performed on a LightSpeed 16 Advantage (16 detectors; GE Healthcare, Milwaukee, Wisconsin) until November 2005 and a LightSpeed VCT (64 detectors; GE Healthcare) thereafter, by using their commercially available software. The parameters for cerebral perfusion imaging were the following: 80 kV, 240 mA, and 0.4-second gantry rotation time; acquisition delay was 7 seconds after injection of 50 mL of iohexol, 300 mg/mL of iodine (Accupaque 300; Amersham, Oslo, Norway) with an injection rate of 5 mL per second into an antecubital vein by using a power injector (Stellant D CT Injection System; Medrad, Indianola, Pennsylvania). Two injections with acquisition of 8 or sixteen 5-mm sections each (four 10-mm sections before October 2005) were performed. The lowest CTP section level usually cut through the thalami before October 2005 and the midbrain thereafter; temporal lobes were therefore variably included. CTP data were transferred to a workstation and analyzed by using different versions of Philips Brain Perfusion software (Philips Medical Systems, Cleveland, Ohio) to create parametric maps of MTT, CBF, and CBV.
For MR imaging, we used both 1.5T (n = 6) and 3T (n = 1) MR imaging systems from different companies and with different acquisition systems. Patients who underwent MR imaging outside our clinic were analyzed either by 0.2T (n = 1) or 1.5T (n = 2) MR imaging systems.
This study was conducted under the auspices of the legislation of the Canton de Vaud, Switzerland, which does not require informed consent for the retrospective scientific analysis of data collected during routine clinical care. In addition, the President of the Ethics Commission for Research on Humans of the Canton and the Medical Director of the institution have granted the right to access medical records to coauthors not directly involved in the care of these patients.
Results
Of 157 patients presenting with clinical symptoms compatible with a diagnosis of TGA between June 2002 and March 2014, 32 (20%) underwent CTP. All examinations were of good quality. Two patients were excluded because of acute ischemic lesions later shown on DWI. Of the remaining 30 patients with a final diagnosis of TGA, 60% were women and the median age was 64 years (interquartile range, 57–70 years). A triggering event (emotional or physical) was documented in 53% of patients. In 77% of the patients who underwent electroencephalography, transient epileptic amnesia was ruled out. In the remaining 23%, there was no clinical suspicion for this diagnosis. Other patient characteristics and the diagnostic tools used are summarized in the Table.
Patient characteristics and diagnostic tests performeda
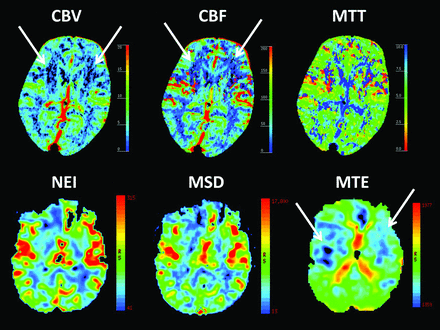
In all except 1 patient, CTP findings were normal, including those of 47% of patients with well-visualized hippocampi. Perfusion abnormalities were observed in a 43-year-old healthy patient with typical TGA presentation. The patient had anterograde and retrograde amnesia spanning several years, accompanied by repetitive questions and moderate anxiety but without identity loss. No triggering event was recorded in this patient. CTP performed at 2.5 hours after symptom onset showed decreased CBF, decreased CBV, and increased MTT in both lentiform nuclei, extending into the insulae but not into the hippocampi. PWI performed 4.0 hours after symptom onset showed findings similar to those of CTP (Fig 1). Acute electroencephalography findings obtained between CTP and MR imaging examinations were normal. When the patient woke up the next morning in the hospital 18 hours after symptom onset, all symptoms had disappeared and no headache, fever, or other symptoms emerged. A neuropsychological assessment performed at approximately 24 hours still showed mild amnestic features (recall difficulties) and a borderline executive dysfunction. The patient was not reassessed with a neuropsychological examination. DWI performed at 4 days did not show any lesions, and repeat CTP findings at 6 days were normal.
Upper row: CTP shows abnormalities in the lentiform nuclei bilaterally (white arrows) in a 43-year-old patient with transient global amnesia at 2.5 hours from symptom onset. Bottom row: PWI shows abnormalities (white arrows) at 4.0 hours from symptom onset. NEI indicates negative enhancement integral (CBV equivalent); MSD, maximum slope of decrease (CBF equivalent); MTE, mean time to enhance (MTT equivalent).
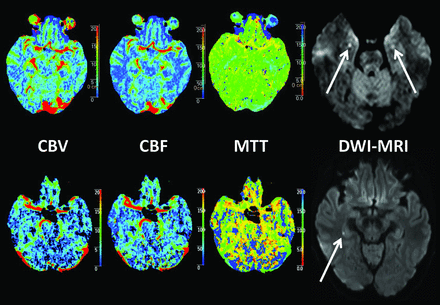
Overall, the frequency of abnormalities found by CTP was 3% in this case series. Low-resolution DWI was performed in 10 of our patients at a median delay of 2 days after symptom onset (interquartile range, 1–3 days). Three of these patients were examined between approximately day 2 and 3, and punctate lesions in the medial hippocampus were observed on DWI in 2 of them 45 and 64 hours after symptom onset, respectively (Fig 2). In the remaining 7 patients outside this time interval, no punctate lesions were detected.
Acute CTP and subacute DWI in 2 female patients presenting with classic TGA symptoms. All CTP map findings for both patients were normal, while punctate DWI lesions were detected in the lateral hippocampus, bilaterally at 45 hours (62-year-old patient, upper row), and unilaterally at 64 hours (61-year-old patient, lower row) after symptom onset, respectively.
Discussion
We found that CTP investigation findings during or immediately after TGA were normal in all except 1 patient in this single-center case series. These results strongly suggest that CTP is not a suitable diagnostic tool to confirm TGA. This finding is also supported by the fact that 2 patients who fulfilled the TGA criteria but had ischemic lesions on MR imaging did not show perfusion abnormalities on CTP.13
Our data do not support widespread hypoperfusion in the mesiotemporal area or elsewhere as a cause of TGA in most patients or of the punctate hippocampal DWI lesions.2,7 Our single patient with CTP perfusion abnormalities showed an unexpected pattern involving both lentiform nuclei and extending into the insulae. We excluded an artifactual finding by confirming the hypoperfusion with an additional PWI within 2 hours and by validating normalization of the CTP 6 days later. These findings are important because on a clinical level, this patient's symptoms were equivalent to those observed in the other patients who lacked perfusion abnormalities.
We do not know whether the CTP flow abnormalities observed could have triggered a punctate lesion in the mesial temporal structures because the hippocampi did not seem to be directly involved and DWI was not performed within the ideal time interval of 48–72 hours after symptom onset.1,2,14 We hypothesize that disruption of the corticohippocampal circuitry without direct involvement of the mesiotemporal region may have caused a clinical syndrome indistinguishable from classic TGA.
In both SPECT and PET studies, perfusion abnormalities in the basal ganglia have been reported for TGA but were associated with concomitant medial temporal lobe hypoperfusion.4,11 Analogous to this study, the case series of patients with TGA examined by using PWI by Toledo et al8 did not show a correlation between perfusion abnormalities and hippocampal DWI lesions on subacute imaging. Hypotheses for such abnormalities temporally related to TGA but lying outside of the limbic system include cortical spreading depression (migraine) or hitherto unexplained mechanisms.
Our study has limitations due to its retrospective nature and the nonconsecutive performance of CTP on the patients with TGA. Furthermore, CTP imaging included the entire hippocampi in only 14 patients, which may have lowered the detection rate of perfusion abnormalities in this region. Only 2 of 10 patients showed typical DWI punctate hippocampal lesions. This result was probably due to the low resolution of our examinations, the use of different MR imaging machines and measurement specifications, and image acquisition outside of the optimal timeframe.
Conclusions
Our data suggest that CTP does not offer additional diagnostic information in the work-up of typical TGA and is therefore not recommended on a routine basis. If imaging is performed in typical or atypical TGA, DWI is the preferred mode of imaging.15
Footnotes
Disclosures: Jean-François Démonet—UNRELATED: Board Membership: Eli Lilly,* Novartis*; Grants/Grants Pending: Leenards Foundation,* European Union Horizon 2020 Small and Medium-sized Enterprises Instrument Phase 2 Alzheimer's Disease Diagnosis by Amoneta Diagnostics project (pending),* project Eurostars Alzheimer's Disease bioCHIP (pending)*; Royalties: Oxford University Press; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Schwabe Pharmaceuticals.* Patrik Michel—RELATED: Grant: Swiss Heart Foundation*; UNRELATED: Board Membership: Boehringer-Ingelheim,* Bayer,* Pfizer*; Consultancy: Amgen,* Pierre-Fabre*; Grants/Grants Pending: Swiss National Science Foundation*; Payment for Lectures (including service on Speakers Bureaus): Boehringer-Ingelheim,* Bayer,* Pfizer,* Covidien,* St. Jude Medical*; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Boehringer-Ingelheim,* Bayer.* *Money paid to the institution.
REFERENCES
- Received January 20, 2015.
- Accepted after revision March 2, 2015.
- © 2015 by American Journal of Neuroradiology