Abstract
BACKGROUND AND PURPOSE: Delayed cerebral ischemia and vasospasm are significant complications following SAH leading to cerebral infarction, functional disability, and death. In recent years, CTA and CTP have been used to increase the detection of delayed cerebral ischemia and vasospasm. Our aim was to perform comparative-effectiveness and cost-effectiveness analyses evaluating CTA and CTP for delayed cerebral ischemia and vasospasm in aneurysmal SAH from a health care payer perspective.
MATERIALS AND METHODS: We developed a decision model comparing CTA and CTP with transcranial Doppler sonography for detection of vasospasm and delayed cerebral ischemia in SAH. The clinical pathways were based on the “Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association” (2012). Outcome health states represented mortality and morbidity according to functional outcomes. Input probabilities of symptoms and serial test results from CTA and CTP, transcranial Doppler ultrasound, and digital subtraction angiography were directly derived from an SAH cohort by using a multinomial logistic regression model. Expected benefits, measured as quality-adjusted life years, and costs, measured in 2012 US dollars, were calculated for each imaging strategy. Univariable, multivariable, and probabilistic sensitivity analyses were performed to determine the independent and combined effect of input parameter uncertainty.
RESULTS: The transcranial Doppler ultrasound strategy yielded 13.62 quality-adjusted life years at a cost of $154,719. The CTA and CTP strategy generated 13.89 quality-adjusted life years at a cost of $147,097, resulting in a gain of 0.27 quality-adjusted life years and cost savings of $7622 over the transcranial Doppler ultrasound strategy. Univariable and multivariable sensitivity analyses indicated that results were robust to plausible input parameter uncertainty. Probabilistic sensitivity analysis results yielded 96.8% of iterations in the right lower quadrant, representing higher benefits and lower costs.
CONCLUSIONS: Our model results suggest that CTA and CTP are the preferred imaging strategy in SAH, compared with transcranial Doppler ultrasound, leading to improved clinical outcomes and lower health care costs.
ABBREVIATIONS:
- CTAP
- CTA and CTP
- DCI
- delayed cerebral ischemia
- ISAT
- International Subarachnoid Aneurysm Trial
- QALY
- quality-adjusted life year
- TCD
- transcranial Doppler ultrasound
- WTP
- willingness to pay
Aneurysmal SAH is a devastating condition resulting in poor clinical outcomes of patients who survive long enough to be admitted, with approximately 15% mortality and 58% functional disability.1 Additionally, as many as 20% of survivors have global cognitive impairment, also contributing to poor functional status.2 Thus, SAH is associated with a substantial burden on health care resources, most of which are related to long-term care.3 Despite advances in techniques for aneurysm repair, poor outcomes remain in SAH partly due to delayed diagnosis and treatment of its secondary complications, mainly vasospasm and delayed cerebral ischemia (DCI).
Currently, there are several methods available to assist with the diagnosis of vasospasm and DCI, including clinical examination, neurologic monitoring devices, transcranial Doppler sonography (TCD), CTA and CTP (CTAP), MR diffusion and perfusion imaging, and digital subtraction angiography. In clinical practice, patients with SAH are primarily assessed by clinical examination and TCD, with clinical examination limited because symptoms are variable and difficult to detect4 and TCD limited by poor sensitivity and specificity.5⇓–7 At the same time, there are studies reported in the literature that support the use of CTAP for detection of both vasospasm and perfusion deficits thought to occur in DCI because of the high sensitivity and specificity of CTAP.8⇓⇓–11 Additionally, emerging data indicate that perfusion imaging may be more accurate for identification of DCI than anatomic imaging of arterial narrowing or changes in blood flow velocity by TCD.8,12 Yet, according to the most recent “Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association” (2012),13 both TCD and perfusion imaging with CT or MR imaging have been assigned the same class IIa recommendation and level B evidence for detection of vasospasm and DCI. Although CTAP has potential to add important diagnostic information for guiding management and treatment decisions, there are no studies to date, to our knowledge, that have assessed the added value of CTAP on clinical outcomes to fully understand its impact in this patient population. Furthermore, there have been no randomized trials comparing the impact of different diagnostic methods on patient outcomes in SAH.
In the past several years, demonstrating the value of imaging has become a major focus in our changing health care environment. Both quality and safety advocates and third-party payers have raised concerns regarding medical practice patterns with inappropriate use of CT, particularly as it relates to radiation exposure. It has become particularly important to substantiate imaging for specific clinical conditions with scientific evidence to guide management and treatment decisions. The purpose of this study was to perform comparative effectiveness and cost-effectiveness analyses evaluating imaging strategies in SAH for detection of vasospasm and DCI by using evidence-based guidelines from a health care payer perspective. Our hypothesis was that CTAP is a cost-effective approach, despite higher imaging costs, compared with the standard imaging strategy using TCD, resulting in improved patient outcomes and averted downstream health care costs.
Materials and Methods
Model Design
We developed a decision model to perform comparative effectiveness and cost-effectiveness analyses by using a decision analytic framework in the TreeAge software program (TreeAge Pro Software, Williamstown, Massachusetts) to compare imaging strategies in SAH from a health care payer perspective. The detailed model structure is provided in On-line Fig 1. CTAP was considered the “new imaging strategy” and was compared with TCD as the “standard imaging strategy.” Clinical examination was included in the model before CTAP or TCD imaging to assess symptoms of vasospasm and/or DCI, as would typically occur in the clinical setting. The model was designed to assess patients during the typical time point at which vasospasm and DCI occur, between 4 and 14 days after SAH.
In the standard strategy, positive concordance between the clinical examination and TCD results led to induced hypertensive therapy, and negative concordance of the clinical examination and TCD led to observation, whereas discordance between the clinical examination and TCD results (such as symptoms but negative TCD findings or no symptoms but positive TCD findings) led to further testing with DSA. In the new strategy, CTA and CTP were evaluated as a single examination termed CTAP because these examinations are typically performed concurrently in clinical practice. If a patient was symptomatic, a positive CTAP finding was defined as a positive result on either CTA or CTP, whereas a negative CTAP finding was defined as a negative result on both CTA and CTP. The rationale is that given the presence of symptoms, a positive result on either examination provides sufficient supportive evidence to prompt treatment decisions. If a patient was asymptomatic, a positive CTAP finding was defined as a positive result on both CTA and CTP, whereas a negative CTAP finding was defined as a negative result on either CTA or CTP. The rationale is that in the absence of symptoms, a single positive result on either examination does not provide sufficient evidence to prompt treatment decisions. All management and treatment decisions are based on the clinical examination and TCD or CTAP combination tests.
The subsequent management and treatment decisions in the clinical pathway incorporated in the model were based on the most recent recommendations in the “Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association” (2012).13 Oral nimodipine was administered to all patients with aneurysmal subarachnoid hemorrhage (class I recommendation, level A evidence) because it has been shown to improve neurologic outcomes after SAH. Treatment of DCI is recommended with induction of hypertension (class I recommendation, level B evidence) and cerebral angioplasty and/or selective intra-arterial vasodilator therapy in symptomatic patients, particularly those who are not rapidly responding to hypertensive therapy (class IIa recommendation, level B evidence).
Each clinical pathway terminated in an outcome health state reflecting the downstream morbidity and mortality. A patient was assigned to only 1 of the 3 following health states: recovered, disability with dependence, or death. The long-term complications of testing or treatment affecting mortality and morbidity (as defined by the health states) were included in the model. Short-term complications such as mild allergic reactions were not included because patients recovered from these temporary ailments; this outcome would not imply significant reductions in quality of life contributing to these health states.
Input Parameter Probabilities
An outcomes-based approach was used in developing the decision model to directly assess the effectiveness of CTAP compared with TCD on clinical outcomes instead of primarily modeling the operating characteristics of these imaging examinations. We modeled probabilities of test results combined with distributions across clinical outcomes, both determined conditional on the clinical examination and imaging results. The probabilities assigned for the occurrence of symptoms and test results of CTAP, TCD, and DSA were directly derived from an SAH cohort enrolled in a diagnostic accuracy study.10 In this cohort, patients had both CTAP and TCD performed, which allowed direct comparison of these imaging strategies in the same patients. According to the study protocol, CTAP was performed at days 6–8 in asymptomatic patients and the same day that symptoms occurred in symptomatic patients with clinical deterioration. Day 7 was the median day CTAP was performed, at which time 45% (44/97) of patients had developed evidence of clinical deterioration.10 Clinical deterioration may manifest by alterations in consciousness, worsening on the Glasgow Coma Scale, or new neurologic deficits. For patients with limited clinical examinations, particularly patients who are comatose or mechanically ventilated, continuous monitoring of laboratory data and neurologic and systemic parameters was used. The details of the CTP scanning protocol and postprocessing methods are provided in the On-line Appendix. TCD was performed daily at the patient's bedside with comparisons between TCD examinations to evaluate changes in blood flow velocity measurements.
Clinical outcomes of functional disability were also assessed in the same SAH cohort10 and obtained from medical record review by a neurologist blinded to the hospitalization course and imaging data. Outcomes were based on the modified Rankin Scale and were categorized into 3 main health states as recovered (mRS 0–2), disability with dependence (mRS 3–5), and death (mRS 6). A multinomial prediction model, incorporating conditional dependence of serial imaging with CTAP and DSA or TCD and DSA, was developed and fitted to determine the predictive probabilities for each outcome based on the test results and on being symptomatic or asymptomatic. The 95% simultaneous confidence intervals for predictive probabilities were computed by using an asymptotic multivariate normal theory and were based on χ2 tests.14 Statistical analysis was performed with “R: A Language and Environment for Statistical Computing” (Version 2.13.0; http://www.r-project.org/).15
Literature sources were used to determine the probabilities of long-term complications from testing (CTAP and DSA) and/or treatment with induced hypertension and intra-arterial (IA) therapy. On-line Table 1 demonstrates these input probabilities and their literature sources.
Assessment of Health Benefits and Costs
Health benefits were measured in quality-adjusted life years (QALYs). For each health state, we assigned a QALY score ranging between 0 and 1.0. Lifetime QALYs were calculated by multiplying the sum of the number of years spent in each health state by the utility associated with that state. The life expectancies were estimated from the literature as 28 years for “recovered”16,17 and 10.8 years for “disability.”18,19 The utilities were calculated as a weighted average from a systematic review of utilities assigned according to mRS scores.20 The utility of “recovered” (mRS 0–2) was 0.80, and it was 0.22 for “disability” (mRS 3–5). By convention, “death” (mRS 6) was assigned a value of zero for both life years and utility.
The economic costs included in the model were those associated with imaging from CTAP, TCD, and DSA; treatment with induced hypertension and IA-therapy; complications from imaging and treatment; and disability after SAH, including both short- and long-term care. The costs for the imaging and treatment were based on the 2012 Medicare reimbursement rates, including both technical and professional fees. The costs for long-term care for patients with stroke have been reported in the literature.21 The estimated cost for recovered patients was calculated as the cumulative annual cost for the expected life years in this health state. The estimated cost for the disability patients was calculated as the first-year rehabilitation costs added to the cumulative annual cost for the remaining expected life years in this health state. By convention, death was assigned no additional cost.
Benefits and costs were discounted at a rate of 3% per year as recommended for cost-effectiveness analyses in the United States.22,23
Model Validation
Internal validity (sometimes referred to as model verification) examines the extent to which the model calculations produced expected outcomes based on the data that were used to derive the model inputs.24 We assessed the internal validity of the model by comparing the predicted probabilities of the health states (recovered, disability, and death) for each imaging strategy in the decision model with the observed outcome data from the SAH cohort. The percentage difference for the model results compared with the observed outcomes was calculated as a measure of deviation for the probabilities of each health state for the standard and new imaging strategies separately.
External validity is performed in a similar fashion but uses outcome data that were not used to develop the model as benchmarks for the predictive performance of the model.24 To evaluate external validity of the decision model, we compared the overall predicted probabilities of the health states for both imaging strategies from the decision model with the published outcome probabilities from the International Subarachnoid Aneurysm Trial (ISAT).1 The percentage difference was also calculated as a measure of deviation for model results relative to published outcomes.
Cost-Effectiveness and Sensitivity Analyses
We calculated the expected benefits and costs associated with each imaging strategy from the perspective of the health care payer. In the primary analysis for policy decision-making, the symptomatic and asymptomatic subgroups were combined in each imaging strategy, weighted by the frequency of symptoms. Additional subgroup analyses were performed to compare the symptomatic and asymptomatic groups separately. An imaging strategy that yielded the greatest quality-adjusted life years and lowest costs would be considered the imaging strategy of choice (dominant strategy).
Sensitivity analyses were performed to test the robustness of the results of the model, given the uncertainty in the input values for the parameters. One-way sensitivity analyses were performed for each parameter by altering the input values across the entire range of possible values (0–1) to identify the key drivers of the model results. In addition, 2-way sensitivity analyses were performed by simultaneously altering the input values for 2 parameters together to assess their combined effects on the results of the model. A willingness-to-pay (WTP) threshold of $100,000 per QALY was used in the 1-way and 2-way sensitivity analyses.
Probabilistic sensitivity analysis was performed to assess the uncertainty in the model results, incorporating the uncertainty of all parameter values together. Distributions for the key model parameters were derived by nonparametric bootstrapping of the SAH cohort,25 focusing attention on the probability of symptoms and the probabilities of imaging (CTAP, TCD, and DSA) conditional on symptoms. Nonparametric bootstrapping was performed by resampling analytic datasets with replacement to evaluate the precision around point estimates without making assumptions regarding the distribution of these variables.26 Parameters that were not directly derived from the cohort (n = 97), such as all other probability inputs, costs, and utilities, were assigned distributions (such as β, uniform, and γ) and varied on the basis of the SD estimates around the mean values. β distribution is a flexible one, which is bounded by 0 and 1, which makes it useful for varying probability inputs in probabilistic sensitivity analysis.26 Uniform distributions assume equal probability of possible random values between 0 and 1.26 γ distributions are rightward skewed with a lower bound at zero, which makes them useful for varying cost inputs in probabilistic sensitivity analysis.26 On-line Table 1 demonstrates the input values and distributions used for all parameters in the model. Results of the probabilistic analysis (10,000 iterations) were used to generate a cost-effective scatterplot and cost-effectiveness acceptability curves to demonstrate the probability that the new strategy is cost-effective for varying cost per QALY thresholds.
Results
Input Parameter Probabilities
On-line Table 1 demonstrates the probabilities of positive and negative test results for CTAP, TCD, and DSA in symptomatic and asymptomatic patients calculated directly from the SAH cohort10 and the predictive probabilities of the outcome health states derived from the multinomial prediction model, incorporating serial imaging and clinical data with conditional dependence.
Model Validation Results
The internal validation revealed that the deviations in the overall probabilities of patients with good (recovered) and poor (disability and death) outcomes estimated from the decision model for the new strategy were 1.2% and 3.4%, respectively, relative to the observed data from the SAH cohort. Within the poor-outcomes group, deviations in the probabilities for disability and death were 0.9% and 13.7%, respectively. For the standard strategy, the deviations in the overall probabilities of patients with good and poor outcomes estimated from the decision model were 5.4% and 16.7%, respectively. Within the poor-outcomes group, deviations in the probabilities for disability and death were 20% and 3.8%, respectively.
The external validation revealed that the deviations in the overall probabilities of patients with good and poor outcomes derived from the decision model were 1.4% and 3.0%, respectively, compared with the published outcomes data from the ISAT.1 Within the poor-outcomes group, deviations in the probabilities for disability and death were 55.3% and 62.2%, respectively.
Base Case Analysis
The Table demonstrates the health benefits (QALYs) and costs for the base case scenario and the subgroup analyses in the symptomatic and asymptomatic patients separately. CTAP resulted in a gain of health benefits with a cost savings in all analyses, thereby dominating the standard imaging strategy.
Health benefits (QALYs) and costs for the base case scenario and subgroup analyses in symptomatic and asymptomatic patients
Sensitivity Analysis
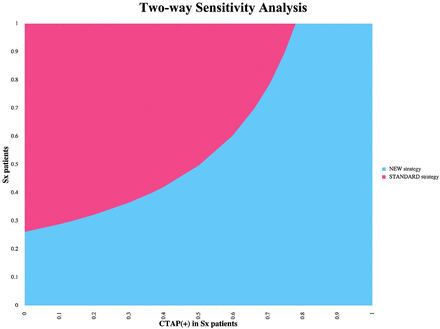
Univariable (1-way) sensitivity analyses indicated that the study results were robust to plausible uncertainty in the parameter values. The CTAP strategy remained preferred (assuming a WTP of $100,000 per QALY) for all parameters varied over the full range of possible input values, except for the following: 1) the probability of positive CTAP fell below 44.2% in symptomatic patients; 2) the probability of complications from induced hypertension rose above 20.5%; and 3) the probability of recovery with negative CTAP findings fell below 51.1% in symptomatic and 85.9% in asymptomatic patients. Multivariable (2-way) sensitivity analyses using the probability of positive CTAP findings in symptomatic patients and the probability of symptoms revealed that the CTAP strategy is dominant when the probability of positive CTAP findings in symptomatic patients is >75% (Fig 1).
Multivariable (2-way) sensitivity analysis graph demonstrating how the decision depends on the values of the probability of positive findings on a CTA and CTP examination in symptomatic patients (Sx) and the probability of symptoms in the SAH population across all ranges. The new strategy represents CTAP imaging, and the standard strategy represents TCD imaging.
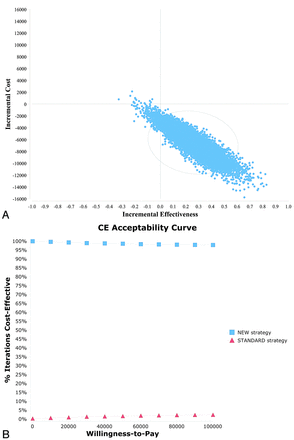
The results of the probabilistic sensitivity analysis (10,000 iterations) were used to generate a cost-effectiveness scatterplot (Fig 2A) and cost-effectiveness acceptability curves (Fig 2B). The cost-effectiveness scatterplot shows that 96.8% of the iterations in the probabilistic sensitivity analysis lie in the right lower quadrant, representing higher effectiveness and lower costs. The cost-effectiveness acceptability curves show that the probability that the new strategy is cost-effective is consistently high for varying cost per QALY thresholds. At the WTP of $50,000 and $100,000 per QALY, the probabilities that the CTAP strategy is preferred are 98.5% and 98.0%, respectively.
Probabilistic sensitivity analysis scatterplot (A) and cost-effectiveness acceptability curves (B) comparing the new strategy versus the standard strategy. The new strategy represents CTAP imaging and the standard strategy represents TCD imaging.
Discussion
Our study reveals that performing CTAP in patients with SAH improves clinical outcomes compared with TCD, with increased quality of life from less functional disability. Although CTAP is associated with higher upfront imaging and treatment costs, there are substantial cost savings associated with this imaging strategy due to overall decreased costs spent on long-term care for patients with functional disability. Our 1-way and probabilistic sensitivity analyses assessed a range of costs for CTAP, from $0 to $2000, and revealed that across these different thresholds, CTAP is still the preferred strategy compared with TCD. CTAP represents a dominant strategy because it provides both better health outcomes and lower costs in the overall patient population and in the symptomatic and asymptomatic subgroups separately. These results support the implementation of CTAP in all SAH, including both symptomatic and asymptomatic patients. Furthermore, our study demonstrates that changing the WTP threshold from $50,000 to $100,000 does not significantly change the model results. The cost-effective acceptability curves generated from the probabilistic sensitivity analysis are helpful in visualizing the effect that a change in WTP can have on these results (Fig 2). CTAP remains the preferred imaging strategy across the entire range of commonly used WTP thresholds in the United States.27
In this cost-effectiveness analysis, an outcomes-based approach was used to directly assess the effectiveness of CTAP compared with TCD on clinical outcomes. Most decision analyses for diagnostic tests model the underlying diagnostic truth in combination with the sensitivity and specificity of the test.27 Here we modeled probabilities of test results combined with distributions across clinical outcomes, both determined conditional on the clinical examination and previous test results. We chose this alternative approach because of controversy about the appropriate reference standard test for DCI and lack of diagnostic performance data of these tests. Because all conditional probabilities of test results and distributions across clinical outcomes were derived from one and the same well-documented cohort of patients with SAH,10 this alternative modeling approach ensures internal consistency of the model. We are confident that the model structure accurately reflects SAH disease pathways because the model results matched the health states (good and poor outcomes) compared with the SAH cohort and ISAT populations in our internal and external model validation analyses. Additionally, the sensitivity analyses demonstrated that the model results were robust to variations in the input values for all parameters.
There are several limitations of our study. First, we modeled health outcomes conditional on diagnostic tests performed in a single cohort, calling into question the generalizability of the model. This approach had the advantage of direct comparison of CTAP and TCD in the same patients, and ensured internal consistency of the data. Even though the external validation of the model revealed that the proportion of patients with good (recovered) and poor (disability and death) outcomes were similar to those in the ISAT,1 greater variation was seen within the poor-outcomes group. The ISAT reported higher mortality compared with the decision model; this would be expected because the older data presented in the ISAT (1994–2002) do not adequately reflect the currently improved mortality rates due to more aggressive management of complications related to SAH such as hydrocephalus, vasospasm, and DCI.13 Additionally, the ISAT reported a 5-year mortality rate rather than the mortality rate within the hospitalization period that we report, which likely also contributed to its higher mortality compared with the decision model because some of our patients may experience delayed mortality due to stroke complications. Furthermore, deviation in the internal validation results was also seen in the poor-outcomes group with the model predicting higher disability and death compared with the SAH cohort. This deviation in the results may be partly attributed to the real-world variation in clinical decision making observed in the SAH cohort compared with the decision model, in which standardized management and treatment decisions were based strictly on the clinical examination and imaging results. Second, the potential long-term effects from low-level radiation exposure used in medical imaging, such as cancer induction, were not included in the model because cancer risks at low doses are very uncertain in the adult population and depend on limited data extrapolating risks from atomic bomb survivors who were exposed to high doses.28 Currently, there are no data available on the lifetime cancer risk from CTAP imaging according to patient age at exposure, sex, and reduced life expectancy from underlying disease, particularly aneurysmal subarachnoid hemorrhage, delayed cerebral ischemia, and/or vasospasm. Because our model was designed to specifically assess patients during the typical time point of DCI and vasospasm between days 4 and 14 after SAH, we only included the morbidity and mortality during the hospitalization period and at discharge. Last, the possible added benefits of performing serial TCD examinations at the bedside to improve detection of vasospasm by monitoring temporal changes in blood-velocity measurements and avoiding intrahospital transportation were not included in the decision analysis because of the lack of estimates in the literature to accurately incorporate them in the model.
Conclusions
In summary, our results have important implications in clinical practice in managing patients with SAH. Our model suggests that CTAP is the preferred initial imaging strategy compared with TCD because it results in both improved clinical outcomes and lower health care costs. Furthermore, the results of this study provide supportive evidence for the widespread implementation of CTAP in both symptomatic and asymptomatic patients. In the current economic environment aimed at improving health care quality and reducing costs, imaging patients with SAH with CTAP should achieve both aims.
Footnotes
Disclosures: Pina C. Sanelli—RELATED: Grant: National Institute of Neurological Disorders and Stroke.* Ajay Gupta—UNRELATED: Grants/Grants Pending: Association of University Radiologists/General Electric Radiology Research Academic Fellowship Award.* Alvin I. Mushlin—UNRELATED: Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Association of University Radiologists/General Electric Radiology Research Academic Fellowship board meeting. *Money paid to the institution.
This work was funded by grant 5K23NS058387 from the National Institute of Neurological Disorders and Stroke, a component of the National Institutes of Health.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official view of National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- Received November 7, 2013.
- Accepted after revision January 25, 2014.
- © 2014 by American Journal of Neuroradiology