Abstract
BACKGROUND AND PURPOSE: The natural history and therapeutic management of dissecting vertebrobasilar aneurysms without ischemic or hemorrhagic stroke (nonstroke dissecting vertebrobasilar aneurysms) are not well-established. We conservatively followed patients with nonstroke dissecting vertebrobasilar aneurysms and evaluated the factors related to clinical and morphologic deterioration.
MATERIALS AND METHODS: One hundred thirteen patients were enrolled and divided by clinical presentation at diagnosis: asymptomatic (group 1, n = 52), pain only (group 2, n = 56), and mass effect (group 3, n = 5). Patients were conservatively managed without intervention and antithrombotic therapy. Clinical outcomes and morphologic changes were analyzed.
RESULTS: A total of 113 patients who were diagnosed with nonstroke dissecting vertebrobasilar aneurysm had a mean follow-up of 2.9 years (range, 27 days to 8 years). Throughout that period, 1 patient in group 1 (1.9%) and 1 patient in group 2 (1.8%) showed clinical deterioration due to mass effect, and 1 patient in group 3 (20%) developed ischemic stroke followed by subarachnoid hemorrhage. Most patients (97.3%) were clinically unchanged. Three patients who had clinical deterioration showed aneurysm enlargement (P < .001). Aneurysms remained morphologically unchanged in 91 patients (80.5%). Aneurysm enlargement was seen in 5 patients (4.4%); risk of enlargement was significantly associated with either maximum diameter (hazard ratio = 1.30; 95% CI, 1.11–11.52; P = .001) or aneurysm ≥10 mm (hazard ratio = 18.0; 95% CI, 1.95–167; P = .011).
CONCLUSIONS: The natural course of these lesions suggests that acute intervention is not always required and close follow-up without antithrombotic therapy is reasonable. Patients with symptoms due to mass effect or aneurysms of >10 mm may require treatment.
ABBREVIATIONS:
- DVBA
- dissecting vertebrobasilar aneurysm
The detection and diagnosis of dissecting vertebrobasilar aneurysms without ischemic or hemorrhagic stroke (nonstroke DVBAs) have increased due to recent advancements in noninvasive brain imaging screening techniques. At the same time, the natural history of nonstroke DVBA is unknown.1,2 Furthermore, the risks and benefits of antiplatelet or anticoagulation therapy for nonstroke DVBA are unclear. The aim of this study was to evaluate the natural course of nonstroke DVBA by identifying the factors associated with clinical and morphologic deterioration and thus contributing to the optimal management strategy.
Materials and Methods
From October 2003 to April 2012, one hundred fifty-five patients at our institution were diagnosed with intracranial DVBA, defined as a nonsaccular aneurysm located at a nonbranching site of the vertebrobasilar artery system. Patients with subarachnoid hemorrhage (n = 11), ischemic stroke (n = 8), prior surgery (n = 11), and a single visit for a second opinion (n = 12) were excluded; thus, 113 patients were enrolled prospectively in this study. It was approved by the institutional review board of our university, and informed consent was obtained from all patients for inclusion.
The 113 patients were divided into 3 groups based on their clinical presentation at diagnosis. Group 1 included asymptomatic patients who were diagnosed incidentally on a prophylactic brain screening MRA study (n = 52), group 2 patients presented with pain only (n = 56), and group 3 patients presented with mass effect from the aneurysm (n = 5). A history of pain was considered relevant only when the patient had a sudden onset of neck or back pain and the date and timing were clearly indicated (Table 1). Patients were followed conservatively with noninvasive imaging studies, without endovascular or surgical intervention. The existence of pre-existing conditions and risk factors, such as hypertension, hyperlipidemia, and current smoking, was evaluated; however, antiplatelet or anticoagulation therapy was not applied in addition to their pre-existing management.
Patient characteristics stratified by clinical presentation at diagnosis
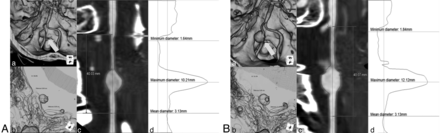
Radiographic evaluation was performed by using 3D-CTA (Somatom Sensation 16; Siemens, Erlangen, Germany) according to our currently accepted protocol. The parameters include FOV = 180, 100 kV, 250 mA, 60-mL contrast at 4 mL/s. The data were transferred to a 3D workstation (TeraRecon, San Mateo, California) by using stretch view software, and the maximum diameter of each aneurysm was measured (including the parent artery, n = 92) (Fig 1). Patients who had contraindications or refused contrast-enhanced CTA (n = 21) were evaluated by MR angiography (Magnetom Symphony 1.5T; Siemens), and their aneurysms were measured by using axial source images from time-of-flight sequences. All 113 patients were observed with consecutive 3D-CTA and/or MRA TOF. Morphologic evaluation classified results as “unchanged,” “improved,” or “enlarged.” “Improved” was defined as any reduction by ≥1 mm in aneurysm diameter or normalization of pearl-and-strings findings (ie, stenosis normalization).
3D-CTA reconstruction on the TeraRecon workstation and stretch view evaluating enlargement of the aneurysm diameter. Initial findings show a maximum diameter, 10.2 mm (A). Follow-up findings after 2 years show an increased maximum diameter, 12.2 mm (B). a, Volume rendering image (arrow indicates an aneurysm). b, Translucent image. c, MIP image of the stretch view. d, Calculated diameter.
Clinical outcomes were evaluated with the modified Rankin Scale, and “deteriorated” was defined as ≥1 point deterioration on the scale.
To find the factors related to clinical and morphologic changes, we also included the following criteria: sex, size (<10 mm versus >10 mm), initial presentation, pre-existing diseases, and smoking history.
Statistical Analysis
Associations between clinical presentation at diagnosis (asymptomatic, pain, and mass effects) and patient characteristics were evaluated by using the χ2 test and analysis of variance. Associations between reduction or enlargement in aneurysm size and clinical presentation at diagnosis and the size of aneurysms and other possible confounders (age, sex, hypertension, diabetes, hyperlipidemia, and smoking status) were evaluated by using the Cox proportional hazards model. Then, significant variables in the models were applied to a multivariate analysis. Two-sided P values < .05 were considered statistically significant. All statistical analyses were performed by using STATA 12.0 (StataCorp, College Station, Texas).
Results
Patient Characteristics
The final cohort consisted of 113 patients, ranging from 31 to 78 years of age (mean, 55 years) and 86 men (76%). The mean follow-up was 2.9 years (range, 27 days to 8 years). Fifty-six (49.6%) patients had a history of hypertension; 51 (45.1%), smoking history; and 23 (20.4%) a history of hyperlipidemia. Patients with hypertension and hyperlipidemia were on medication according to their physicians' prescriptions. Patients in group 1 were significantly older than those in group two: 59.3 years versus 51.1 years, respectively. Hypertension was more prevalent in group 1 than in the other 2 groups.
Clinical Findings
Of 113 patients, 3 deteriorated during the study period, and the incidence of clinical deterioration was significantly higher in group 3 (P = .048). One patient in group 3 (0.9%) developed an ischemic stroke (Wallenberg syndrome) and was admitted to another institution where the patient received anticoagulation therapy. Subsequently, the patient had SAH (Hunt and Hess grade 2) and was transferred back to our institution, where endovascular obliteration was performed. Two patients (1.8%), 1 in group 1 and 1 in group 2, experienced new clinical symptoms due to mass effect (Tables 1 and 2).
Cases with radiologic and clinical deterioration
Radiologic Findings
Radiologic evaluation of aneurysm size revealed a statistically significant difference (P < .001) among the 3 groups: In most of the patients in group 1 (96.2%), aneurysm size remained unchanged, in contrast to 26.8% of patients in group 2% and 20% in group 3 in whom aneurysm size became smaller.
On the basis of proportional hazards models, pain as a clinical presentation at diagnosis significantly increased the chance for aneurysm reduction. In contrast, larger maximum aneurysm diameter and hypertension at diagnosis were associated with less possibility of aneurysm reduction. Using an adjustment with significant variables, a multiple regression analysis found that pain and aneurysm size at diagnosis were still significantly associated with aneurysm reduction (Table 3).
Cox proportional hazards model to find factors associated with aneurysm reduction
Next, similar to our analysis of the factors associated with aneurysm reduction, we analyzed the factors associated with aneurysm enlargement. The risk of aneurysm enlargement was significantly associated with either maximum diameter (hazard ratio = 1.30; 95% CI, 1.11–11.52; P = .001) or aneurysm of ≥10 mm (hazard ratio = 18.0; 95% CI, 1.95–167; P = .011) (Table 4). All 3 patients who clinically deteriorated showed enlarged aneurysms, and that association was statistically significant (P < .001), though the number was small.
Cox proportional hazards model to find factors associated with aneurysm enlargement
Discussion
Management Strategy and Clinical Findings
Reports of incidentally found DVBAs are rare, but the detection of DVBAs is rising due to increased and improved screening by brain imaging. Therapeutic management of patients with DVBA who are asymptomatic or present only with pain remains controversial and is not well-established.2,3 DVBA with pain is considered a critical condition predictive of stroke, for which either a surgical or endovascular approach is the treatment of choice.4⇓–6 Rabinov et al7 reported on 28 patients with DVBA who were treated surgically or endovascularly; 2 patients presented with pain only, of whom 1 was surgically treated and rated a score of 3 on the modified Rankin Scale. Ahn et al5 reported their endovascular treatment of 14 patients with DVBA, of whom 5 presented with headache only. In our series, none of the patients who presented with pain only at diagnosis had a hemorrhagic or ischemic stroke during observation. On the basis of our results, aggressive interventional treatment may not be necessary for patients with DVBA presenting with pain only.
The establishment of optimal medical management is also controversial. Kim et al8 reported their large series of 191 patients with unruptured vertebrobasilar artery dissections, including ischemic and nonischemic symptoms, in which 81 patients presented with headache only. All were treated either endovasculary (n = 46) or by medical therapy with anticoagulation (n = 49), antiplatelet therapy (n = 48), or analgesics (n = 48). The necessity for anticoagulation or antiplatelet therapy remains unclear; our results, at least, do not prove the necessity for anticoagulation or antiplatelet therapy for patients presenting with pain only.
We did not use any antiplatelet or anticoagulation therapy during observation. One patient who presented with mass effect at diagnosis had a brain stem infarction during observation and was admitted to another hospital. The patient received anticoagulation therapy and subsequently experienced SAH. Some similarities can be found with Tsutsumi et al,9 who reported a vertebral artery dissection causing SAH in a similar patient who had initially presented with infarction and had received antiplatelet agent therapy. Thus, pain management and blood pressure control are recommended for patients presenting with pain only or ischemic symptoms.
Yasui et al10 reported their histopathologic findings of incidentally detected fusiform vertebrobasilar aneurysms, which demonstrated intimal thickening, disruption of the internal elastic lamina, and degeneration of the media. They concluded that an incidental fusiform vertebrobasilar aneurysm has the potential to develop into a dissecting aneurysm. In our series, only 1 patient with an incidentally found DVBA experienced clinical deterioration and aneurysm growth during observation.
Morphologic Changes
Observation with serial radiologic examinations should be performed on patients with unruptured DVBAs. Most of the DVBAs in group 1 did not show morphologic findings in the vessel wall. Sato et al11 showed that disrupted internal elastic lamina, covered with intimal thickening, is commonly found at postmortem examination in normal intracranial vertebral arteries of patients who died of causes other than intracranial lesions. Most of the incidentally detected lesions occurred silently or with minor headache and vessel wall healing occurred silently.
Ahn et al12 reported the difference in the morphologic evolution of the lesion; 25 of 34 symptomatic intracranial vertebrobasilar dissections with dilation without stenosis had no change compared with their initial shape. Pozzati et al13 described cases of spontaneous resolution of fusiform aneurysm in the vertebrobasilar system proved by angiography. Naito et al1 reported that angiographic features of vertebral artery dissection changed for 12 of 20 patients (60%) and that deterioration of features was seen in 4 cases (20%). Nakagawa et al14 reported serial angiographic changes in 88.2% of unruptured vertebral artery dissections. In our series, morphologic changes were observed in 22 of 113 patients (19.5%) and 5 (4.4%) had DVBAs that enlarged. As for DVBAs having the possibility of changing morphologically, we suggest serial radiologic follow-up for nonstroke DVBAs.
Mizutani15 reported on 44 patients who presented with nonischemic unruptured dissections in the vertebrobasilar system, of which 10 DVBAs improved, 4 enlarged, and 1 ruptured during observation. Mangrum et al16 reported that enlargement of nonsaccular intracranial aneurysms correlated significantly with median aneurysm diameter and symptomatic compression at diagnosis. Flemming et al17 reported that the annual prospective risk of hemorrhage from a vertebrobasilar artery nonsaccular intracranial aneurysm is 0.9% and that an aneurysm diameter of at least 10 mm is strongly indicative of future rupture. In our series, aneurysms of <10 mm had a favorable clinical outcome, but aneurysms of >10 mm with symptoms due to mass effect had a risk of clinical deterioration and enlargement. In such cases, surgical or endovascular intervention should be considered.
Limitations of the Study
This study has several limitations. First, the radiologic examination findings were not the same for all patients. The diameter of the aneurysm was different between CTA and MRA. Nevertheless, the goal of this study was not to compare the differences in the devices but to evaluate the changes in findings with the same method. Each patient was evaluated with either CTA or MRA TOF only. Second, we defined “DVBA” as a nonsaccular aneurysm located at a nonbranching site of the vertebrobasilar artery; however, it is sometimes difficult to determine whether an incidental asymptomatic fusiform aneurysm may have resulted from spontaneously healed dissections or other underlying vascular abnormalities, especially with only CT. Third, intramural hematoma was significantly associated with a change of vertebrobasilar dissection on follow-up in a previous report.12 In this study, most imaging was performed with CT angiography. CTA has limitations in detecting an intramural hematoma. Fourth, in this study, mean follow-up was 2.9 years. This time is relatively short to evaluate the natural history of these lesions. In this study group, 3 patients demonstrated relatively good clinical outcome, though these patients showed significant risk of enlargement. This discrepancy may be due to lack of long-term follow-up. Fifth, in some cases, follow-up was discontinued, and these patients might have experienced SAH or infarction and might have been admitted elsewhere. Finally, we have a small number of patients with nonstroke DVBA who were treated with endovascular therapy; they do not entirely reflect the results of the study, but this small number will have a negligible effect on the natural course of nonstroke DVBA for the whole group. Although the study has these limitations, the results may provide important information for the treatment and further investigation of nonstroke DVBA.
Conclusions
The natural course of nonstroke DVBA is favorable during a 2.9-year period. The short-term course of these lesions suggests that acute intervention is not always required and close follow-up is reasonable, unless patients develop symptoms associated with significant mass effect. Patients with symptoms due to mass effect or the size of the aneurysm (diameter of >10 mm) may deteriorate and eventually require intervention.
Acknowledgments
The authors acknowledge the assistance of Naoya Kunikane, RT, Yukiko Abe, RT, and Hiroto Narita, PhD, with technical support for 3D-CTA.
Footnotes
Disclosures: Yuichi Murayama—UNRELATED: Consultancy: Asahi Intecc, Stryker, Grants/Grants Pending: Siemens,* Stryker,* Asahi Intecc,* Fujifilm,* Patents (planned, pending, or issued): Stryker,* Royalties: Stryker.* Ichiro Yuki—UNRELATED: Grants/Grants Pending: Siemens.* Toshihiro Ishibashi—UNRELATED: Consultancy: Stryker Japan. Hiroyuki Takao—UNRELATED: Grants/Grants Pending: Fujifilm,* NTT DoCoMo.* Ikki Kajiwara—UNRELATED: Grants/Grants Pending: Siemens.* Mitsuyoshi Urashima—UNRELATED: Grants/Grants Pending: Ministry of Education, Culture, Sports, Science, and Technology in Japan, a supported program for the Strategic Research Foundation at private universities.* *Money paid to the institution.
References
- Received August 30, 2013.
- Accepted after revision December 2, 2013.
- © 2014 by American Journal of Neuroradiology