Abstract
BACKGROUND AND PURPOSE: Acute intracranial hemorrhage represents a severe and time critical pathology that requires precise and quick diagnosis, mainly by performing a CT scan. The purpose of this study was to compare image quality and intracranial hemorrhage conspicuity in brain CT with sinogram-affirmed iterative reconstruction and filtered back-projection reconstruction techniques at standard (340 mAs) and low-dose tube current levels (260 mAs).
MATERIALS AND METHODS: A total of 94 consecutive patients with intracranial hemorrhage received CT scans either with standard or low-dose protocol by random assignment. Group 1 (n=54; mean age, 64 ± 20 years) received CT at 340 mAs, and group 2 (n=40; mean age, 57 ± 23 years) received CT at 260 mAs. Images of both groups were reconstructed with filtered back-projection reconstruction and 5 iterative strengths (S1–S5) and ranked blind by 2 radiologists for image quality and intracranial hemorrhage on a 5-point scale. Image noise, SNR, dose-length product (mGycm), and mean effective dose (mSv) were calculated.
RESULTS: In both groups, image quality and intracranial hemorrhage conspicuity were rated subjectively with an excellent/good image quality. A higher strength of sinogram-affirmed iterative reconstruction showed an increase in image quality with a difference to filtered back-projection reconstruction (P < .05). Subjective rating showed the best score of image quality and intracranial hemorrhage conspicuity achieved through S3/S4–5. Objective analysis of image quality showed in an increase of SNR with a higher strength of sinogram-affirmed iterative reconstruction. Patients in group 2 (mean: 744 mGycm/1.71 mSv) were exposed to a significantly lower dose than those in group 1 (mean: 1045 mGycm/2.40 mSv, P < .01).
CONCLUSIONS: S3 provides better image quality and visualization of intracranial hemorrhage in brain CT at 260 mAs. Dose reduction by almost one-third is possible without significant loss in diagnostic quality.
ABBREVIATIONS:
- DLP
- dose-length product
- FBP
- filtered back-projection reconstruction
- HU
- Hounsfield units
- ICH
- intracranial hemorrhage
- SAFIRE
- sinogram-affirmed iterative reconstruction
At present, CT of the brain is the imaging technique of choice for evaluation of an intracranial hemorrhage (ICH). CT imaging adds valuable information regarding the extent and severity of an ICH. Every effort should be made to accurately detect ICH because of the higher mortality rate without treatment.
CT examinations account for only a minority of radiologic procedures but represent a significant portion of the radiation dose received from all medical procedures.1⇓⇓⇓⇓–6 Because of the potential radiation risk through ionizing radiation and because CT is frequently in use for patients with head trauma, every effort should be made to keep the dose as low as reasonably achievable.
Many approaches to reduce patient dose have been investigated including routine use of automated exposure control software, and reduction of tube current and tube potential. Reducing the tube current is eventually limited by increased noise leading to a decrease in image quality. Recently, iterative reconstruction techniques for CT have been introduced to decrease image noise as an alternative to the standard filtered back-projection (FBP) method.7⇓⇓⇓–11 Earlier versions of iterative reconstruction algorithms required a high amount of computational calculating time and could not be used in this form for emergency radiologic procedures.12 The second generation of iterative reconstruction processes, sinogram-affirmed iterative reconstruction (SAFIRE), is now commercially available. SAFIRE estimates the noise content in raw data caused by fluctuations in neighboring voxels and subtracts the noise stepwise in several validation loops. The result of the first correction loop is compared with the “master data,” and an updated image is generated for the next iteration, leading to further noise reduction. Offered by various vendors, this technique should be able to reduce the necessary radiation dose by 35%–76% while maintaining equivalent image quality.13⇓–15 For this purpose, we conducted this study to compare the SAFIRE algorithm and FBP regarding image quality and detectability of ICH and reduction of radiation dose in brain CT scanning.
Materials and Methods
Patient Selection
This study was approved by our institutional review board. The data analyzed in this study were acquired in a timeframe of 8 months. In this timeframe, all patients referred for CT of the brain were examined either with the standard CT protocol (group 1) or with the new protocol with reduced mAs (group 2) by random assignment. All consecutive patients with ICH were included: 54 patients from group 1 and 40 patients from group 2.
Examination Techniques
All patients underwent the examination on multidetector row CT scanners (Somatom Definition Flash/AS; Siemens, Erlangen, Germany). CT protocol settings followed the manufacturer's recommendations. The parameters were kept constant, except the tube current–time products; 340 mAs was the standard scan parameter and 260 mAs was the new parameter for this study, as recommended by the manufacturer. The CT was acquired in axial image orientation, 48-mm detector coverage (4 × 20 × 0.6 mm), a small field of view, and 120 kVp. Images from the top to the base of the anterior cranial fossa were evaluated.
Image Reconstruction
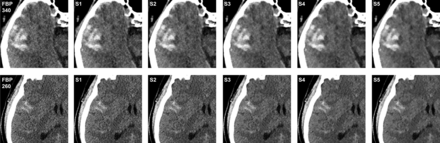
Images of both tube current levels (340 mAs and 260 mAs) were reconstructed with a medium smooth kernel (H30s, J30s) into 4-mm sections by use of FBP and 5 different blending strengths of SAFIRE (S1–S5), which led to a total of 6 image datasets as shown in Fig 1. All image sets were sent for processing to a PACS workstation (Centricity 4.1; GE Healthcare, Dornstadt, Germany).
CT images of a 67-year-old man with intracerebral bleeding acquired with tube current–time product of 340 mAs (upper row) and a 48-year-old man with subarachnoid hemorrhage acquired with tube current–time product of 260 mAs (lower row), each with FBP and 5-strength of the SAFIRE technique.
Dose Estimates
For the estimation of radiation doses, we recorded the dose-length product (DLP in mGycm) and the effective tube current-time product (effective mAs) from the patient protocol, which is automatically generated after the end of an examination and stored in the PACS of our department. The effective dose was calculated as the product of DLP and the normalized value of effective dose per DLP for the head (0.0023 mSv mGy-1 cm-1; European guideline on quality criteria for CT, European Commission, EUR 16262).
Subjective Image Quality
All CT image datasets were displayed in random order on a diagnostic monitor for the assessment of subjective image quality and with all images displayed on constant window settings (window width, 80 Hounsfield units [HU]; window level, 40 HU). These datasets were reviewed by 2 radiologists with experience in neuroradiology in a blinded manner. The overall image quality of brain structures and the image quality and identifiable properties of cerebral hemorrhage were ranked by use of a 5-point scale (1 = worst image quality, 2 = fair image quality, 3 = moderate image quality, 4 = good image quality, 5 = best image quality). The subjective image quality ratings from FBP and SAFIRE S1–S5 at each level of tube current–time product were compared with standard FBP at 340 mAs and FBP at 260 mAs.
Objective Analysis of Image Quality
As measures of image quality, 4 ROI measurements were performed on a PACS workstation by use of a circle tool with a diameter of 3–6 mm for the ROIs. The measurements were performed by a radiologist with 1 year of experience in CT of the brain. Image noise (IN) was determined as the standard deviation of air in the level of the frontal lobe. Mean attenuation values (A) and standard deviation were measured in the GM and WM in the superior frontal gyrus and in the center of the ICH: cerebral, epidural, subdural, and subarachnoid hemorrhage. The measurements for 6 image datasets of each patient were recorded and displayed in HU. On the basis of these measurements, SNR was determined according to the following equation:
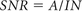
The objective image quality ratings from FBP and SAFIRE S1–S5 at each level of tube current–time product were compared with standard FBP at 340 mAs and FBP at 260 mAs.
Statistical Analysis
Computer-based statistical analyses were performed with dedicated software (BiAS 9.17; Epsilon, Frankfurt, Germany). Patient age, image noise, SNR, subjective image quality, and DLP were expressed as mean values and standard deviations. Age and DLP were tested by use of the Wilcoxon-Mann-Whitney U test. For SNR and subjective image quality rating, the Fisher exact test with a Bonferroni-corrected P value was used. A P value of < 5% was considered to be statistically significant. Interobserver agreement of subjective image quality rating was assessed with the Cohen weighted κ analysis. Definitions of levels of agreement on the basis of κ values were as follows: κ < 0.3 indicated slight agreement; κ = 0.3–0.7, moderate agreement; and κ > 0.7 meant good agreement.
Results
Patient Characteristics
In group 1 with the standard protocol, 54 patients with a mean age of 64 ± 20 years (age range, 17–90 years) were included. In group 2, with a reduced tube current–time product of 260 mAs, 40 patients with a mean age of 57 ± 23 years (age range, 16–95 years) were included. No significant differences regarding age were found concerning patient characteristics.
Subjective Image Quality Measurements
Subjective image quality was rated for FBP and SAFIRE S1–S5 at each level of tube current–time product. Data are summarized in Table 1. Subjective overall image quality of brain structures was rated with excellent interobserver agreement for both readers for group 1 at 340 mAs (κ = 0.91) and group 2 with 260 mAs (κ = 0.80). In a likewise fashion, the image quality with visibility of ICH in group 1 and group 2 was rated subjectively with a good interobserver agreement (κ = 0.92 and κ = 0.9, respectively). The score for image quality of brain structures increased with a higher strength of SAFIRE at each level of tube current–time product up to S3 and for image quality of ICH up to S4–S5. The best score for the image quality of brain structures was achieved with SAFIRE S3 with significant difference to the FBP reconstruction in each group (P < .05). The best score for the visualization of ICH was achieved with SAFIRE S4 and S5 with significant difference to the FBP reconstruction in each group (P < .05). The difference between standard FBP at 340 mAs and SAFIRE 1 blending at 260 mAs had no predominant statistical significance for image quality of the brain structures and ICH (P > .05). When compared with 1 reconstruction technique on its own (FBP or SAFIRE S1–S5) between both tube current–time products, there was no statistically significant difference (P > .1) for S3–S5 depicting brain structures and for S1–S5 depicting ICH conspicuity.
Subjective image quality rating (1 = worst, 5 = best) for brain structures and intracranial hemorrhage for filtered back-projection and 5 strengths (S1–S5) of sinogram-affirmed iterative reconstruction technique
Objective Image Quality Measurements
Statistical results of the objective image quality measurements are summarized in Table 2. Image noise was higher (P > .05) in group 2 (260 mAs) than in group 1 (340 mAs) for all reconstruction techniques. Image noise decreased with higher strength of SAFIRE; therefore, SNR increased with a higher strength of SAFIRE at each level of tube current–time product (mAs). The difference between FBP and SAFIRE 1 was statistically significant (P < .05) for SNR GM and ICH at 340 mAs, whereas no significant difference was reached for examination at 260 mAs. No statistically significant difference was shown for SNR WM at SAFIRE 1 for both groups. SNR of SAFIRE 3 up to 5 at 260 mAs and 340 mAs was higher than FBP at each level of tube current–time product (P < .05).The highest SNR was reached with SAFIRE 5 in each group. When compared with 1 reconstruction technique on its own (FBP or SAFIRE S1–S5) between both tube current–time products, there was no statistically significant difference (P > .05) for FBP, S1 and S2 depicting WM, and FBP and S2 depicting ICH.
Objective image quality measurements with SNR and image noise for filtered back-projection and 5 strength (S1–S5) of sinogram-affirmed iterative reconstruction technique
Radiation Dose
Patients examined with a tube current of 260 mAs were exposed to significantly less radiation dose than the group examined with 340 mAs (260 mAs: mean DLP, 744 ± 80 mGycm; 340 mAs: mean DLP, 1045 ± 108 mGycm; P < .01). Even the calculated mean effective dose was lower at 1.71 mSv (260 mAs) compared with 2.40 mSv (340 mAs).
Discussion
Previous studies have applied the standard deviation to assess the objective image quality.8,16 To assess the objective image quality in the brain and to compare the level of the signal with the level of background noise, we applied the SNR of selected ROIs to WM and GM and the region with ICH as a measurement of objective image quality. Ren et al17 showed a possible reduction of the tube current–time product down to 200 mAs on CT of the brain with the adaptive statistical iterative reconstruction technique without focus on ICH. Because of ethical reasons, we followed the manufacturer's recommendations. With reduction of the tube current–time product from the standard 340 mAs down to 260 mAs, the mean effective dose decreased from 2.40–1.71 mSv, which results in a relevant reduction of 29%, higher than the 20.4% calculated by Korn et al,18 and similar to Ren et al17 and Kilic et al19 with 30% and 31% dose reductions, respectively.
Compared with the FBP reconstruction technique, all iterative reconstruction techniques increased SNR heterogeneously by 13%–88%, depending on the algorithm strength and tube current–time product. These results are similar to those of Schulz et al12 for CT of the paranasal sinus and Leipsic et al9 for coronary CT angiography. There was no significant difference for SNR between FBP and SAFIRE S1 for group 2 at 260 mAs. Therefore, we concluded that SAFIRE 1 could not provide a better noise reduction than FBP in lower-dose examination at 260 mAs. We came to the conclusion that SAFIRE 3–5 is able to reduce noise and increase objective image quality in standard brain CT even with a lower tube current–time product.
SAFIRE 3 was rated best for overall image quality of the brain at both mAs levels, with the best κ value of 0.62 for examination at 260 mAs. SAFIRE 4–5 was rated best for the visualization of ICH, and we conclude that the higher noise reduction leads to a better demarcation of lesions with rich contrast. The use of the SAFIRE 1 reconstruction technique with a reduced tube current–time product of 260 mAs could achieve the same image quality as a standard examination at 340 mAs. SNR at 340 mAs is still higher than in low-dose examination at 260 mAs, but it does not affect radiologic diagnosis significantly. For standard use, we recommend a protocol at 260 mAs with an iterative algorithm. The benefit of higher SNR at 340 mAs can be assumed for postoperative or therapeutic cerebral status. An appropriate examination protocol should be reserved for patients with a relevant medical history.
Although the SNR increases with a higher strength of SAFIRE, the subjective image quality with SAFIRE S4 and S5 is worse than with SAFIRE S3. We deduce that a high SAFIRE strength does not necessarily imply a good image quality. This phenomenon is similar to what has been reported in previous studies. Increased image blurring has been discussed in several publications that investigated iterative reconstruction techniques.9,12,14,20,21 Silva et al20 suggested that the diminished noise manifests as an oversmoothing of the images. Singh et al8 thought that higher blending proportions of iterative reconstruction to FBP could substantially change the texture and characteristics of the images. As discussed before, SAFIRE offers a better reconstruction technique for the detection of ICH than FBP, and it can be assumed that a better reconstruction technique with SAFIRE reconstruction leads to a lower false-negative value of the detection of ICH, better treatment, and a possible reduction of radiation risk. Although the κ value varied for the subjective image qualities, the variation tendency of the 2 radiologists was consistent, probably because of different diagnostic experience and different understanding about the scales. These differences cannot be avoided, but in a randomized and blinded manner, they can be minimized.
For the future, iterative algorithms should be used for the detection of ICH. This should be accompanied by a lowering of the false-negative rate for the detection of ICH. SAFIRE 3 is the choice for evaluation of brain structures and SAFIRE 5, the choice for evaluation of ICH at both mAs levels. Inexperienced readers are recommended to use SAFIRE 3 for evaluation of the brain structures and SAFIRE 5 for evaluation and detection of ICH in combination. To optimize the processing time, experienced readers should rely on the SAFIRE 3 algorithm at 260 mAs for evaluation of the brain structures and detection of ICH with 1 reconstruction algorithm. The benefit of SAFIRE 5 compared with SAFIRE 3 concerning the conspicuity of ICH would not affect radiologic diagnosis significantly for experienced readers.
There were limitations to our study. First, we could not perform an intrapatient comparison, but we had 2 groups with no significant difference regarding age, and all patients were referred for assessment of ICH. Second, the measurement by ROI did not provide information for the whole brain. However, with essential ROIs we received valid results as evidence for the subjective image quality in the whole brain. For future studies, SAFIRE can be used in combination with other dose-reduction techniques, for instance, automatic tube-current modulation, as proposed by Smith et al22 as an effective dose-reduction method or automatic tube-voltage modulation.
Conclusions
SAFIRE improves image quality and visualization of ICH on head CT with a normal-dose and a low-dose protocol. For standard use, we recommend a protocol at 260 mAs with a SAFIRE algorithm with the benefit of a reduction in radiation dose by approximately 29%. For evaluation in patients with postoperative or therapeutic cerebral status, a benefit at 340 mAs can be assumed. SAFIRE 3 showed an increased image quality for evaluation of brain structures and SAFIRE 5 for ICH conspicuity compared with the FBP reconstruction technique at both mAs levels. The use of SAFIRE 3 at 260 mAs is recommended for evaluation of brain structures and detection of ICH, optimizing processing time for experienced readers. For unexperienced readers, SAFIRE 5 at 260 mAs should be used for evaluation of ICH in combination with SAFIRE 3 for evaluation of brain structures. SAFIRE should be used to diminish the false-negative rate. Therefore, a better detection with an iterative algorithm can result in better treatment.
Footnotes
Disclosures: Ralf Bauer—UNRELATED: Payment for Lectures (including service on speaker bureaus): Seimens AG. J. Matthias Kerl—UNRELATED: Payment for Lectures (including service on speaker bureaus): Siemens AG. J. Matthias Kerl and Ralf Bauer are consultants for Siemens Medical Care.
Paper previously presented in part at: European Congress of Radiology, March 7–11, 2013; Vienna, Austria.
REFERENCES
- Received March 18, 2013.
- Accepted after revision July 8, 2013.
- © 2014 by American Journal of Neuroradiology