Abstract
SUMMARY: Cystic parenchymal lesions may pose an important diagnostic challenge, particularly when encountered in unexpected locations. Dilated perivascular spaces, which may mimic cystic neoplasms, are known to occur in the inferior basal ganglia and mesencephalothalamic regions; a focal preference within the subcortical white matter has not been reported. This series describes 15 cases of patients with cystic lesions within the subcortical white matter of the anterior superior temporal lobe, which followed a CSF signal; were located adjacent to a subarachnoid space; demonstrated variable surrounding signal change; and, in those that were followed up, showed stability. Pathology study results obtained in 1 patient demonstrated chronic gliosis surrounding innumerable dilated perivascular spaces. These findings suggest that dilated perivascular spaces may exhibit a regional preference for the subcortical white matter of the anterior superior temporal lobe. Other features—lack of clinical symptoms, proximity to the subarachnoid space, identification of an adjacent vessel, and stability with time—may help in confidently making the prospective diagnosis of a dilated perivascular space, thereby preventing unnecessary invasive management.
ABBREVIATION:
- SAS
- subarachnoid space
Cystic lesions of the brain parenchyma pose an important diagnostic challenge, because the differentiation between benign and malignant lesions is often difficult on neuroimaging alone. In the classic sense, the differential diagnosis includes benign acquired lesions (enlarged perivascular spaces or Virchow-Robin spaces, porencephalic cysts or cystic encephalomalacia, chronic lacunar infarctions, or parasitic cysts), benign congenital lesions (neuroglial cysts or ependymal cysts), and cystic neoplasms.
Dilated Virchow-Robin spaces are expansions of the normal perivascular space that may mimic a cystic neoplasm. Previous literature has demonstrated a predilection for involvement of the mesencephalothalamic region,1 and although these structures may involve the subcortical white matter of the cerebral hemispheres, this location is less common. A focal preference within the subcortical white matter has not been reported previously.
At our institution, we identified 15 sequential cases of cystic lesions within the subcortical white matter of the anterior superior temporal lobes, all demonstrating nearly identical location and morphologic and imaging features. Here, we discuss their imaging characteristics and differential diagnosis, and propose that these lesions may be representative of a newly identified preferential location for a dilated perivascular space.
Case Series
Case Selection
We obtained institutional research ethics board approval for this study. A total of 15 consecutive cases were identified at our institution with the presence of a subcortical cystic lesion within the anterior superior temporal gyrus, with imaging performed between November 2011 and January 2013. The corresponding patient records for each case were retrospectively reviewed for prior imaging studies indicating imaging follow-up. Information on demographic features, clinical symptoms prompting initial imaging, and any subsequent development of neurologic symptoms was also noted.
Imaging Acquisition
All imaging studies were performed on either a 1.5T Signa Excite HD (GE Healthcare, Milwaukee, Wisconsin) or a 1.5T Magnetom Avanto or 3T Magnetom Verio (Siemens, Erlangen, Germany) MR imaging scanner. Sequences performed in all MR imaging acquisitions included sagittal T1, axial FLAIR, and DWI sequences. Depending on the scanner used, either a gradient-echo sequence or a susceptibility-weighted sequence was obtained. T2 sequences were occasionally performed (these are not routinely performed at our institution in follow-up examinations of previously recognized lesions). Gadolinium-enhanced imaging was obtained in all patients within the period of follow-up.
Results
In all 15 cases, the lesions demonstrated signal characteristics following that of CSF on all sequences (Figs 1 and 2; Table 1). All lesions were noted to closely appose the adjacent subarachnoid space (SAS). Most lesions (13/15) were elongated morphologically. None of the lesions showed associated enhancement, restricted diffusion, or susceptibility artifacts throughout the follow-up period. In those with imaging follow-up, no change in size was noted in any patient throughout the follow-up period (range, 6–112 months), either involving the lesion itself, or the degree of surrounding signal change. Finally, all lesions except for 1 lesion (identified in case 7) demonstrated either no perilesional FLAIR/T2 signal change, or mild perilesional signal change. On the basis of these findings, the diagnosis of a dilated perivascular space was suggested in each case, with a limited differential diagnosis provided. Of note, 2 patients (cases 6 and 11) had multiple adjacent, smaller, linearly-oriented lesions that followed a CSF signal on all sequences—these were thought to represent smaller prominent Virchow-Robin spaces (Fig 3). In case 7, there was extensive surrounding perilesional signal change, thought to represent perilesional edema; this patient ultimately underwent surgical resection because of suspicion of a low-grade neoplasm (Fig 1C).
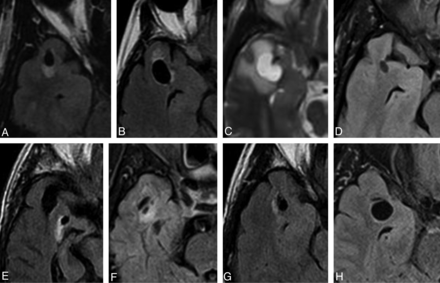
Selected axial FLAIR/T2 images of right-sided lesions within the anterior superior temporal gyrus as identified in case 1 (A), 2 (B), 7 (C), 8 (D), 9 (E), 10 (F), 12 (G), and 14 (H). All lesions were located adjacent to the SAS. The images illustrate the variability in the degree of surrounding signal change. Note the proximity to the adjacent middle cerebral artery and more prominent surrounding signal hyperintensity seen in case 7 (C).
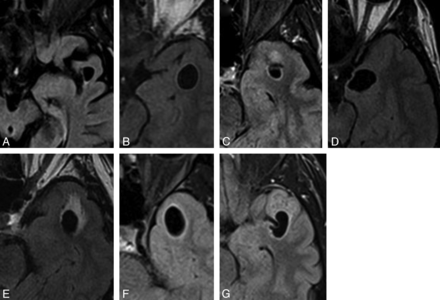
Selected axial FLAIR images of left-sided lesions within the anterior superior temporal gyrus as identified in case 3 (A), 4 (B), 5 (C), 6 (D), 11 (E),13 (F), and 15 (G). All lesions were located adjacent to the SAS. Again, the images illustrate the variability in the degree of surrounding signal change.
MR imaging characteristics of lesions identified within the anterior superior temporal subcortical white matter
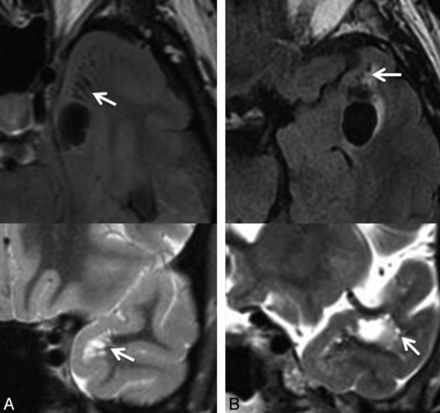
Selected axial FLAIR images demonstrating the presence of a cystic lesion within the anterior temporal gyrus as identified in case 6 (A) and case 11 (B), with adjacent smaller cystic lesions, suggestive of a dominant enlarged perivascular space with adjacent smaller prominent perivascular spaces (arrows). Corresponding coronal T2 images (below) through the region of interest confirm these findings (arrows). In case 11 (B), the proximity to the adjacent middle cerebral artery is identified on the coronal T2 image.
Corresponding clinical findings for each patient were also obtained (On-line Table). In most cases, on the basis of neurologic or neurosurgical evaluation, the lesions were felt to be incidental or were not felt to account for the patients' presenting symptoms (headache, etc). However, in 2 patients (cases 2 and 7) the presenting clinical symptoms could potentially have been attributed to the identified lesion. In case 2, the patient presented with seizures typical of temporal lobe epilepsy localized to the right temporal region, coinciding with the location of the cystic subcortical lesion. On the basis of the clinical work-up, it was thought plausible that these seizures might be arising from the lesion with potential associated cortical irritation; however, the patient remained seizure-free while receiving medication, and given the stability of the imaging findings and clinical course, surgical management was not indicated. For the patient in case 7, the combination of the clinical presentation suggesting possible temporal lobe seizures and the atypical imaging feature of extensive perilesional signal change prompted surgical management, following which the patient's symptoms completely resolved.
As a result, pathology study was obtained in only 1 patient (case 7). Results demonstrated a region of chronic demyelination and gliosis surrounding innumerable dilated perivascular spaces, which, aside from the dominant large perivascular space, were presumably below the resolution of MR imaging (Fig 4).
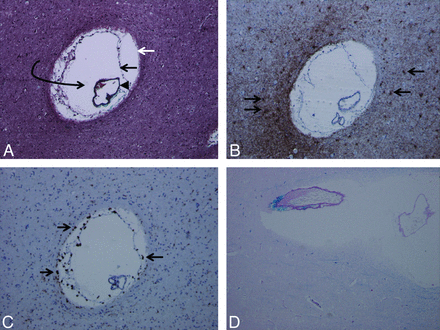
Trichrome stain (A) demonstrates the presence of an enlarged perivascular space (white arrow, glia limitans; black arrow, inner pial membrane; black arrowhead, vessel lined by outer pial membrane; curved arrow, perivascular space). Multiple such lesions were seen in the surgical specimen. Glial fibrillary acidic protein stain (B) and CD68 stain (C) demonstrate reactive astrocytes (arrows in B) and perivascular microglia (arrows in C) as multiple brown-staining dots, confirming chronicity of the pathophysiologic process. Luxol fast blue stain (D) demonstrates diffuse absence of blue staining (myelin staining), indicating demyelination and gliosis in the brain parenchyma surrounding the dilated perivascular space (magnification of all slides, 20×).
Discussion
Perivascular spaces are leptomeningeal-lined spaces that surround penetrating arteries as they course within the brain parenchyma. The leptomeningeal layers that line these spaces create a small, fluid-filled space around the arterial wall that is distinct from the subpial and SAS, allowing for a slightly different fluid composition than that present in CSF. These structures are usually identified in normal, healthy patients on high-resolution MR imaging and often demonstrate a curvilinear morphologic pattern along the trajectory of penetrating vessels.2
Dilated perivascular spaces are benign expansions of these normal structures, typically located at the inferior basal ganglia along the anterior commissure.3 Giant perivascular spaces, measuring 1.5 cm, most commonly occur within the mesencephalothalamic region, where they may manifest with obstructive hydrocephalus.1 Although less common, these lesions have been reported to exist focally elsewhere in the brain parenchyma, including the subcortical white matter.1 Regardless of specific location, enlarged perivascular spaces have been noted to abut either the ventricular margin or the SAS, as seen in the cases of all of our patients.1 Diffusely enlarged perivascular spaces have also been reported, which have been hypothesized to occur in the context of certain pathologic conditions, including chronic microvascular ischemia, Alzheimer dementia, cerebral amyloid angiopathy, CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), and mucopolysaccharidoses.4⇓⇓⇓–8
Regarding the function of perivascular spaces, several theories have been proposed. They are thought to play a role in immunologic processes in the brain, to provide a drainage route for interstitial fluid, and to provide a mechanism for equalization of intracranial pressure.9 However, the cause of their enlargement, and the reason for regional predilection to dilation, is poorly understood. Proposed mechanisms of enlargement include those related to the associated vessels, namely arterial elongation or increased vascular permeability; those related to the surrounding brain parenchyma, such as volume loss with ex vacuo enlargement; or those related to CSF or interstitial fluid dynamics, including altered or increased CSF pulsations with increased pressure within the perivascular space, or obstruction to flow of interstitial fluid on the basis of amyloid deposition.1 However, the mechanisms by which these processes may occur focally in some cases, and diffusely in others, remain unexplained.
Previous literature on dilated perivascular spaces has demonstrated the tendency of these lesions to exhibit a regional preference. The largest series of dilated perivascular spaces, reported by Salzman et al,1 described 37 cases, of which 21 (57%) were located in the mesencephalothalamic region, whereas only 8 (22%) were within the subcortical white matter. It is interesting to note that of all lesions located within the white matter, 50% exhibited surrounding perilesional signal change on FLAIR/T2 sequences, but none of the mesencephalothalamic lesions demonstrated this associated finding. This supports our findings because most of our cases demonstrated perilesional signal alteration. However, Salzman et al1 did not describe a temporal predominance of the subcortical lesions because only 2 of the white matter lesions were identified within the temporal lobe. A smaller series reported by Cerase et al10 described 3 cases of dilated perivascular spaces where the lesions regressed with time: All were located in the anterior superior temporal lobe, corresponding exactly to the location we describe in our series, and one of the lesions exhibited perilesional signal change on FLAIR sequence.
The cause of perilesional signal change has also been discussed previously, with a proposed theory being that of accelerated white matter ischemic change resulting from compression of the adjacent parenchymal vessels by the enlarged perivascular space.11 A more interesting theory, however, and one that is reflected in our MR imaging and pathologic findings, is that the high signal may be the result of gliosis in addition to multiple prominent perivascular spaces that are below the resolution of MR imaging.1,12 Although we were able to prospectively identify prominent perivascular spaces surrounding the dominant lesion in 2 patients only (cases 6 and 11), our pathologic specimen was far more revealing. It is interesting to note that our patient (case 7) underwent surgical excision partially on the basis of MR imaging findings that suggested perilesional vasogenic edema, which was demonstrated on pathologic findings to be nothing more than the presence of multiple prominent perivascular spaces amid diffuse gliosis.
The ultimate objective of studying perivascular spaces is to elucidate imaging features that may help distinguish these benign lesions from more ominous pathologic conditions in a prospective manner. The predilection for certain locations is an important distinguishing feature of dilated perivascular spaces. Although previously only reported to favor the mesencephalothalamic region, our findings, in conjunction with several other reported cases, propose that the anterior superior temporal subcortical white matter is an additional preferential site for dilated perivascular spaces. Other features may also be helpful in identification of these structures, including their orientation and shape, which may often be elongated or fusiform in morphologic appearance, suggesting their association with a penetrating vessel.13 In our cases, the proximity to the SAS with the identification of the adjacent middle cerebral artery is an additional attribute that helps to distinguish these lesions, such that they may be associated with the course of a perforating vessel. Finally, the lack of associated enhancement or restricted diffusion, as well as the stability of these lesions with time, also act as key indicators of the underlying pathologic condition. Our cases, as well as those reported in the literature, demonstrate that perilesional FLAIR/T2 signal change may be identified in the context of dilated perivascular spaces and should not, in itself, prompt alternative diagnoses to be entertained.
It is important to note that only 5 of our patients had adequate imaging follow-up (range, 22–112 months) to indicate stability with time. Four patients had follow-up ranging from 6–14 months, and 5 patients did not undergo imaging follow-up at our institution (1 patient [case 7] without follow-up underwent surgery). For the cases that did not undergo imaging follow-up, one cannot rely on imaging stability to aid in diagnosis; however, given that these patients were asymptomatic regarding the lesion, and all other suggestive imaging features were present, including location within the anterior superior temporal lobe, the diagnosis of dilated perivascular spaces can be strongly suspected. In these situations in which the diagnosis of dilated perivascular spaces is highly favored but not proven, surgical management may still be avoided in favor of a more conservative approach.
Other benign lesions in the differential diagnosis that may have a similar imaging appearance include neuroglial or glioependymal cysts, which include the subtype of ependymal cysts. These rare lesions are thought to develop from sequestered embryologic remnants of neuroectoderm or neural tube elements.14 Although they demonstrate regional preferences—ependymal cysts are commonly identified in a juxtaventricular location, whereas other neuroglial cysts demonstrate frontal lobe predominance—these locations do not coincide with that of dilated perivascular spaces, thereby providing another basis on which to distinguish these pathologic conditions.14,15 Acquired lesions, on the other hand, including porencephalic cysts, cystic encephalomalacia, or sequelae of lacunar infarctions, would not be expected to demonstrate a regional preference, given that they are the result of prior insults to the brain that would not necessarily occur in a specific location. Finally, infectious cysts and cystic neoplasms would not necessarily follow a CSF signal on all sequences, could demonstrate associated enhancement, and would be expected to enlarge or change with time.
Conclusions
This series of cases suggests that dilated perivascular spaces may exhibit a regional preference for the subcortical white matter of the anterior superior temporal lobe. In addition to location, other imaging features—including proximity to the SAS, identification of an adjacent vessel, absence of enhancement or restricted diffusion, and stability with time—may help in confidently making the diagnosis of a dilated perivascular space, thereby preventing unnecessary invasive management. The presence of a perilesional FLAIR/T2 signal change should not exclude this diagnosis, because this feature may be present in the context of a dilated perivascular space and does not necessarily suggest a more worrisome pathologic condition.
References
- Received April 2, 2013.
- Accepted after revision May 9, 2013.
- © 2014 by American Journal of Neuroradiology