Abstract
BACKGROUND AND PURPOSE: Stroke is a leading cause of death and disability, and many studies have focused on the evolution of FLAIR imaging in the acute and chronic time window. The purpose of this study was to evaluate the potential efficacy of FLAIR-related techniques in identifying the onset time of cerebral ischemia in a canine embolic stroke model.
MATERIALS AND METHODS: An embolic ischemic model was generated through the use of an autologous clot in 20 beagle dogs. Both FLAIR and DWI were performed at 3 hours, 4 hours, 5 hours, 6 hours, and 24 hours after embolization, respectively. Visual “DWI-FLAIR mismatch” was defined as hyperintense signal detected on DWI but not on FLAIR. The relative signal intensity of FLAIR-positive lesions and the degree of DWI-FLAIR mismatch was calculated as relative FLAIR = relative signal intensity of FLAIR positive lesions, mismatch degree = (100−VFLAIR/VDWI) × 100%.
RESULTS: The ischemic model was successfully established in all animals. FLAIR-positive lesions were seen in 3, 11, 16, 19, and 20 beagle dogs at 5 time points after embolization, respectively. There was significant correlation between the relative FLAIR, degree of DWI-FLAIR mismatch, and the onset time (relative FLAIR: r = +0.42; 95% CI, 0.20–0.60; mismatch degree: r = −0.85; 95% CI, 0.89–0.78). Receiver operating characteristic curves showed that the degree of DWI-FLAIR mismatch could identify the hyperacute ischemic lesions with a sensitivity range from 1.00–0.76; visual DWI-FLAIR mismatch sensitivity ranged from 0.85–0.39, whereas specificity was 0.83–0.95 versus 0.85–1.00.
CONCLUSIONS: The relative FLAIR and DWI-FLAIR mismatch values were useful in predicting the onset time in our canine embolic stroke model. The degree of DWI-FLAIR mismatch proposed in our study could be a good indicator with high sensitivity for identifying the hyperacute ischemic stroke.
ABBREVIATIONS:
- rSI
- relative signal intensity
- DWI-FLAIR mismatch
- DWI positive and FLAIR negative
- ROC
- receiver operating characteristic
- rFLAIR
- relative signal intensity of FLAIR positive lesions
- SI
- signal intensity
- rADC
- relative ADC
Intravenous administration of tPA is the only proven, effective treatment for acute ischemic stroke within the first 4.5 hours after onset of symptoms.1,2 However, an estimated 25% of ischemic strokes occur during sleep, and the exact time of the onset of symptoms is unclear, which means that a large group of patients is precluded from the time-based thrombolytic therapy, especially in developing countries.3 Therefore, a new diagnostic method that can identify the onset time of stroke is urgently required.
Recently, FLAIR sequences of MR imaging in acute stroke have attracted more attention as a potential surrogate marker for time since stroke onset.4,5 Specifically, a visual mismatch between DWI and FLAIR images has been shown to identify patients likely to be within a time window of 4.5 hours.6⇓–8 Although these findings showed the high specificity of a “DWI-FLAIR mismatch” as a “tissue clock” to identify patients potentially eligible for thrombolytic therapy, some limitations of the method remained, such as different interobserver agreement rate, patient selection bias, and especially the relatively low sensitivity.
To overcome these limitations, we performed several quantitative measurements with the use of FLAIR images, including relative signal intensity (rSI) of FLAIR lesions, degree of DWI-FLAIR mismatch, and also visual DWI-FLAIR mismatch, to evaluate the potential efficacy of FLAIR-related techniques and to identify the onset time of acute cerebral ischemia in a beagle dog model.9,10
Material and Methods
Animal Preparation and Model Establishment
The surgical procedures and experimental protocol were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (Nanjing, China). Effective actions were taken for reducing pain or discomfort during the experiments.
A total of 20 adult healthy beagle dogs of either sex, weighing 12–15 kg, were anesthetized with intravenous injection of 3 mg/kg of pentobarbital (Pentobarbital Sodium Salt, Chemical Reagent Company, Shanghai, China). The airways were secured by means of oral endotracheal tubes with spontaneous respiration. Bilateral femoral arterial and left femoral venous accesses were obtained by use of 5F sheaths for catheterization, physiologic monitoring, and drug administration. Body temperature was maintained at 37–39°C during the interventional procedures and the duration of recovery after procedures by use of heating blankets. Sterile procedures were strictly used in all cases.
The beagle dog's cerebral ischemic models were established through the use of a method similar to the one used in our previous reports.9,10 Briefly, a prepared autologous clot (approximately 1.7 mm in diameter and 5 mm in length) was injected into the left proximal MCA under live fluoroscopy, and embolization was confirmed by angiography. After that, a 5F catheter was guided to 2 cm distal to the orifice of the ipsilateral ICA to block the blood flow for 2 hours. The animals were then transported to the MR imaging suite for imaging studies.
MR Imaging
Imaging was performed with a 3T MR system (Magnetom Trio; Siemens, Erlangen, Germany), with the use of a transmit-receive extremity coil with a diameter of 15 cm. Imaging acquisitions were performed serially at 3 hours, 4 hours, 5 hours, 6 hours, and 24 hours after the left MCA embolization, respectively. The same medications and doses were used to maintain immobility and sleep during the MR imaging scan. We checked T2-weighted images (acquisition matrix = 320 × 320, TR = 5000 ms, TE = 76 ms), FLAIR (acquisition matrix = 320 × 320, TR = 8000 ms, TE = 97 ms), and DWI (acquisition matrix = 320 × 320, TR = 5500 ms, TE = 97.3 ms). After MR examination, all animals recovered and were kept in the animal facility for other studies.
Imaging Assessment
FLAIR-positive (FLAIR+) or DWI-positive (DWI+) was defined as new hyperintense signals detected on FLAIR or DWI. For signal intensity (SI) changes of ischemic lesions on FLAIR, an ROI was defined on 1–3 sections showing the most obvious lesion completely covering the ischemic area. Control SI values were obtained from an ROI drawn contralaterally. Two independent raters (Y. Sheng, Q.G. Cheng) judged FLAIR+ according to acute lesions on DWI. Relative SI was obtained from FLAIR images according to the following formula: rSI = lesion SI/contralateral SI.
For ADC changes of ischemic lesion on DWI, an ROI was also defined on the sections showing the most obvious lesion. Control ADC values were obtained from an ROI drawn contralaterally. The relative ADC (rADC) value was obtained according to the following formula: rADC = lesion ADC/contralateral ADC.
“DWI-FLAIR mismatch” was defined as new hyperintense signal detected on DWI but not on FLAIR. The sensitivity and specificity values were calculated for the allocation of dogs to time interval from symptom onset to MR imaging scan within 3 hours, 4 hours, 5 hours, and 6 hours by DWI-FLAIR mismatch.
The volume of DWI+ and FLAIR+ lesions at each time point were also calculated. For the quantification of lesion size on DWI and FLAIR, the lesions in the same section were delineated by 3 authors (X.-Q. Xu, S.-S. Lu, Q.-Q. Zu), with consensus by use of an operator-defined ROI on each of the lesion-containing sections. The lesion volumes were obtained by multiplying the lesion areas by the section and gap thickness. Through the use of the volume of DWI+ and FLAIR+ lesions, the degree of DWI-FLAIR mismatch was calculated according to the following formula: Degree = (100−VFLAIR/VDWI) × 100%. During the whole imaging assessment process, if any discrepant results occurred between 2 evaluators, the third and senior evaluator (S. Liu) would make the final decision.
Statistical Analysis
Interobserver and intraobserver agreement for the rating of FLAIR, DWI, and ADC images was assessed by means of Pearson correlation coefficient. The correlation between rFLAIR and onset time of stroke, and the correlation between the degree of DWI-FLAIR mismatch and the onset time were assessed by Spearman ρ analysis. The linear regression curve was plotted by use of GraphPad Prism statistical analysis software (GraphPad Software, San Diego, California). An optimal cutoff value was determined from the receiver operating characteristic (ROC) curve to analyze if there were critical ADC values that were sensitive or specific for FLAIR positivity. An ROC curve was used to identify the optimal cutoff value to allocate the ischemic lesions within 3 hours, 4 hours, 5 hours, and 6 hours with degree of DWI-FLAIR mismatch. The numeric data were averaged over all animals and reported as mean ± standard deviation. A significant difference was considered if the P value was <.05. Statistical analysis was carried out with SPSS 17.0 (IBM, Armonk, New York).
Results
Ischemic Model
All 20 cerebral ischemic models were established successfully without any procedure-related complications or casualties (Fig 1). DWI+ lesions were seen in all 20 beagle dogs starting from 3 hours after embolization. The DWI indicated that the cerebral ischemic lesions were located on the ipsilateral caudate nucleus and the cortical area of the temporal lobe. Generally, the ischemic lesions were first found at the caudate nucleus, followed by the lesions located in the cortical area of the temporal lobe (Fig 2).
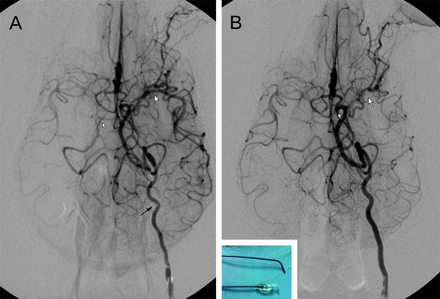
Representative real-time DSA images before and after MCA embolization in beagle dogs. A, Angiography clearly demonstrated the anatomy of intracranial arteries, including the left MCA trunk (large arrowhead), tortuous ICA (black arrowhead), and cerebral circulation (small arrowhead) through the left ICA injection before embolization. B, At the arterial phase, angiography immediately after thrombus injection through the catheter, the main trunk of MCA was completely occluded (small and large arrowheads). After that, a 5F catheter was guided to 2 cm distal to the orifice of ipsilateral ICA to block the blood flow for 2 hours. The catheter used in our study is displayed in left-bottom inset.
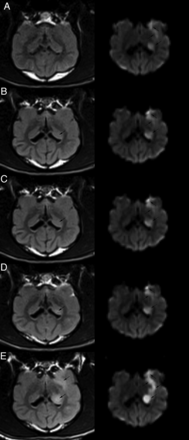
Sequential DWI and FLAIR images of coronal sections. The DWI and FLAIR were performed at 3 hours (A), 4 hours (B), 5 hours (C), 6 hours (D), and 24 hours (E) after embolization, respectively. There was no signal abnormality at 3 hours on the FLAIR image (A, left). Relative slightly high signal intensity area (arrow) with ovoid shape was observed in the left caudate nucleus on 4-hour FLAIR image (B, left). Increased high signal intensity was seen at 5 hours, 6 hours, and 24 hours on the FLAIR image (C–E; left), and lesions in the cortical area were seen on the 24-hour FLAIR image (E, left). Sequential DWI of coronal sections was all positive from 3 hours to 24 hours after model establishment (A–E; right).
Correlations Between rFLAIR with Time after the Model Was Established
Intraobserver agreement for qualitative judgment of FLAIR lesion visibility of observer 1 and observer 2 was 87% (k = 0.78; 95% CI, 0.77–0.98) and 85% (k = 0.77; 95% CI, 0.75–0.96), respectively. Intraobserver agreement for quantitative judgment of FLAIR+ lesions of observer 1 and observer 2 was 89% (k = 0.81; 95% CI, 0.80–0.99) and 91% (k = 0.84; 95% CI, 0.82–0.98), respectively.
Interobserver agreement for qualitative judgment of FLAIR lesion visibility was 80.1% (k = 0.52; 95% CI, 0.56–0.91). Interobserver agreement for quantitative judgment of rFLAIR lesion was 86% (k = 0.77; 95% CI, 0.76–0.98).
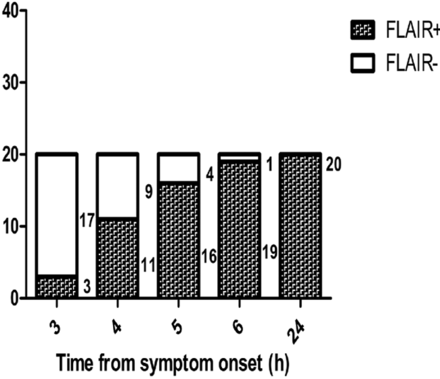
FLAIR+ lesions were seen in 3 of the 20 (15%) beagles at 3 hours after embolization, 11 of the 20 (55%) beagles at 4 hours after embolization, 16 of the 20 (80%) beagles at 5 hours after embolization, 19 of the 20 (95%) beagles at 6 hours after embolization, and all 20 (100%) beagles at 24 hours after embolization, respectively (Fig 3).
Proportion of FLAIR-negative and FLAIR-positive results on the basis of the 5 onset time points in the 20 beagle dogs. Number of FLAIR-negative and FLAIR-positive dogs are presented in bars.
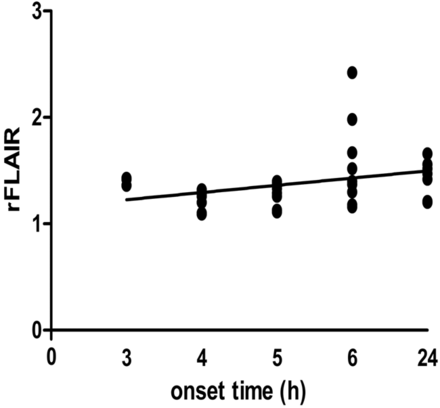
All 69 FLAIR+ lesions had a mean rSI of 1.40 ± 0.27. The rFLAIR values were 1.40 ± 0.04, 1.23 ± 0.13, 1.27 ± 0.03, 1.59 ± 0.09, and 1.43 ± 0.07 at 3 hours, 4 hours, 5 hours, 6 hours, and 24 hours after embolization, respectively. In a linear regression model, there were significant correlations between rFLAIR lesions and onset time (P < .05). The Spearman correlation coefficient for rFLAIR and onset time was +0.42 (95% CI, 0.20–0.60) (Fig 4).
Relative SI on FLAIR images and time interval from onset to MR imaging scanning. Significant correlation between the rFLAIR and the onset time was found (r = +0.42; 95% CI, 0.20–0.90).
Correlation Between FLAIR+ Images and the rADC Threshold Value
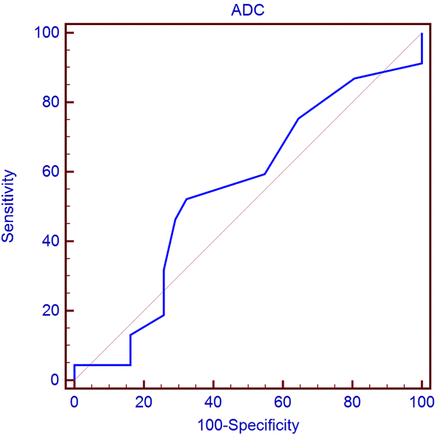
The ROC curve analysis results indicated that the rADC value of 0.54 might be the critical threshold value. With the rADC value of 0.54 set as the diagnostic threshold value, the best sensitivity and specificity for judging FLAIR positivity were 0.52 and 0.68, respectively (Fig 5).
ROC of the rADC value in identifying the FLAIR+ lesions. ROC curve showed that the rADC value of 0.54 might be the critical threshold value that could quantify FLAIR+ lesions with the optimal sensitivity and specificity (0.52 and 0.68, respectively).
Application of DWI-FLAIR Mismatch in the Ischemic Model
According to the pattern of DWI-FLAIR mismatch, the interval time between onset and image acquisition was presumed to be within 3 hours, sensitivity of 0.85 and specificity of 0.85; within 4 hours, sensitivity of 0.65 and specificity of 0.92; within 5 hours, sensitivity of 0.50 and specificity of 0.97; and within 6 hours, sensitivity of 0.39 and specificity of 1.00.
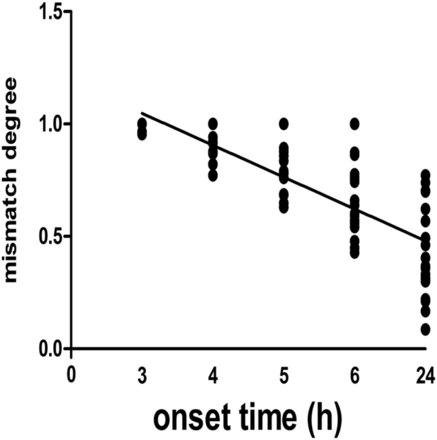
Meanwhile, the degree of DWI-FLAIR mismatch was also calculated by means of the formula: degree = (100−VFLAIR/VDWI) × 100%. Interobserver agreement for quantitative judgment of “degree of mismatch” was 79% (k = 0.77; 95% CI, 0.72–0.84). The degree of DWI-FLAIR mismatch was 0.99 ± 0.00, 0.93 ± 0.01, 0.81 ± 0.11, 0.67 ± 0.06, and 0.42 ± 0.10 at 3 hours, 4 hours, 5 hours, 6 hours, and 24 hours after embolization, respectively. In a linear regression model, there is a significant correlation between degree of DWI-FLAIR mismatch and onset time (P < .05). The Spearman correlation coefficient for degree of DWI-FLAIR mismatch and onset time was −0.85 (95% CI, 0.89–0.78) (Fig 6).
Degree of DWI-FLAIR mismatch and time interval from onset to MR imaging scanning. Significant correlation between the degree of DWI-FLAIR mismatch and the onset time was found (r = −0.85; 95% CI, 0.89–0.78).
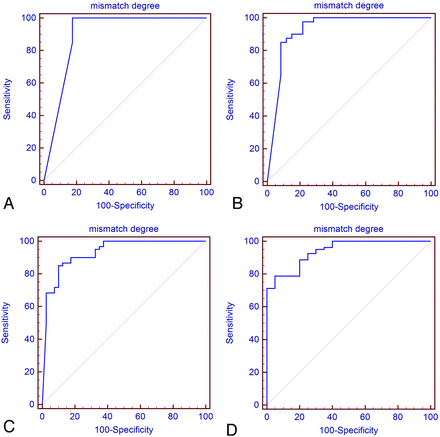
The ROC analysis results indicated that the mismatch degree value of 0.95 might be the critical threshold value to identify the ischemic lesion within 3 hours, with a sensitivity and specificity of 100% and 82.5%, respectively. The mismatch degree value of 0.90 might be the critical threshold value to identify the ischemic lesion within 4 hours, with a sensitivity and specificity of 85% and 91.7%, respectively. The mismatch degree value of 0.79 might be the critical threshold value to identify the ischemic lesion within 5 hours, with a sensitivity and specificity of 83.3% and 90%, respectively. The mismatch degree value of 0.75 might be the critical threshold value to identify the ischemic lesion within 6 hours, with a sensitivity and specificity of 76.2% and 95%, respectively (Fig 7).
ROC of the DWI-FLAIR mismatch in identifying the hyperacute ischemic lesions within different onset time points. ROC showed that the cutoff values of DWI-FLAIR mismatch degree were 0.95, 0.90, 0.79, and 0.75, respectively, giving sensitivity of 1.00, 0.85, 0.83, and 0.76 for identifying the hyperacute ischemic lesion at 3 hours, 4 hours, 5 hours, and 6 hours after embolization, respectively.
Discussion
Our study demonstrates several major findings. First, the sensitivity of FLAIR for detecting hyperacute ischemic lesions clearly increases over time after embolization. Second, there is a significant correlation between the rFLAIR and the interval time from ischemic onset to MR imaging scanning. Thus, the rSI may be useful to predict the onset time of ischemic stroke. Third, also the most important, the new parameter, the degree of DWI-FLAIR mismatch proposed in our study, could be a better indicator for identifying the hyperacute ischemic stroke than visual DWI-FLAIR mismatch. To our knowledge, this is the first study to test the potential efficacy of a FLAIR-related technique, especially DWI-FLAIR mismatch and degree of DWI-FLAIR mismatch in identifying the onset time of cerebral ischemia on the basis of an ischemic stroke animal model with known onset time. Our study design can effectively avoid the selection bias as reported by previous retrospective clinical studies.
Whether rFLAIR can serve as an indicator to predict the onset time of cerebral ischemia is still debated. Ebinger et al11 insisted that there was no significant correlation between rFLAIR and the onset interval time (r = −0.15, P = .128), after analyzing 102 FLAIR+ and 203 DWI+ lesions of 94 consecutive patients. However, Cheng et al12 declared that there was a moderately significant correlation between the rFLAIR and onset interval time (r = 0.38, P < .001), which is similar to opinions of Petkova et al13 (r = 0.63, P < .001). Interestingly, the correlation ratio in our study was located between that of the latter 2 studies. Discrepancies in the results of correlations may partly be explained by patient characteristics. The median volume of FLAIR+ lesions in the Cheng et al study was larger than that of the Ebinger et al study (4.5–10.5 mL versus 0.86–1.65 mL). As a matter of course, the NIHSS score in the Cheng et al study was higher than that of the Ebinger et al study (7.3–8.8 versus 3–4). The larger ischemic volume and higher NIHSS score sometimes mean longer intervals in time from onset to MR imaging; therefore, more patients would be in the later time interval. Furthermore the statistical results would be different consequentially. However, in the Cheng et al study, data analysis was performed on images from multiple centers. This condition might lead to a heterogeneous set of FLAIR sequence parameters, which might contribute to a decreased homogeneity of rFLAIR, thus explaining a lesser correlation than that reported by Petkova et al13 and our study. Altogether, we think that a perspective, multiple center study with the uniform sequence parameters was needed to verify whether rSI of the FLAIR+ lesions can serve as an alternative indicator to predict the onset time of cerebral ischemia.
Recently, a visual mismatch between DWI and FLAIR in acute stroke has attracted increased attention. Previous studies indicated that DWI-FLAIR mismatch could serve as a surrogate marker of lesion age.6⇓–8 In our study, by use of this mismatch pattern to identify the hyperacute lesions within 3 hours, 4 hours, 5 hours, and 6 hours, we recorded specificities of 0.85, 0.92, 0.97, and 1.00, respectively, which were similar to those in previous studies. As we know, onset time of acute ischemic stroke is critical for guidance of thrombolysis therapy.1,14,15 Earlier management sometimes means better prognosis and fewer complications.16 However, if patients whose onset time is beyond the time window receive thrombolytics therapy, the risk of intracranial hemorrhage would increase and the thrombolysis therapy would be harmful or even lethal. Because of this, high specificity of the pattern of DWI-FLAIR mismatch appears to be crucial in improving the safety of the thrombolytic therapy, especially for patients with unknown symptom onset. However, also similar to the previous study, the sensitivity of the use of DWI-FLAIR mismatch to identify the hyperacute lesions is relatively low (0.39–0.85). So, if we choose the visual DWI-FLAIR mismatch as the criteria for identifying the acute stroke patients, only 39% of the patients (within 6 hours) would meet the criteria. As a result, more than half (61%) of the patients would lose the chance of thrombolytic therapy.
Considering that visual DWI and FLAIR mismatch is a binary concept—and the varied interobserver agreement rate among previous studies—our study proposes the utility of a new quantitative parameter, degree of DWI-FLAIR mismatch, for determining the onset time of stroke. With the use of the new index, we found that there were significant correlations between degree of DWI-FLAIR mismatch and onset time (r = −0.85; 95% CI, 0.78–0.89). Meanwhile, by use of the optimal mismatch degree threshold identified by ROC analysis to identify the hyperacute ischemic lesions, we acquired markedly enhanced sensitivity compared with the initial visual mismatch analysis. At the same time, an increase of sensitivity did not result in an obvious decrease in specificity. Why the new mismatch index could effectively enhance the sensitivity is, we think, because the visual DWI-FLAIR mismatch represents the concept of “all or nothing.” According to the criteria of visual DWI-FLAIR mismatch, there are just 2 results (“mismatch” or “no mismatch”), regardless of the ischemic lesions volume. Therefore, in our study, we transformed the binary DWI-FLAIR concept to a quantitative mismatch degree. The new mismatch degree index could effectively narrow the differences between each individual and each time point, rectify the influence of the lesion volume, and thus capture the difference that was omitted by the simple “yes or no” concept.
DWI and FLAIR techniques are more effective in identifying hyperacute strokes. First, we spent only approximately 45 seconds and 3 minutes, 8 seconds, on DWI and FLAIR techniques, respectively. In general, approximately 4 minutes was needed to get the DWI and FLAIR image information. Considering the time spent on image processing and analysis, this could save more time in identifying patients with hyperacute stroke than some other functional MR modalities. Second, both DWI and FLAIR techniques are contrast-free image modalities, which make them safer and with fewer contrast-related complications in an emergency.
There are still several limitations in our study that should be discussed. First, the ischemic model established in our study resembles the situation of a tandem occlusion in humans that will seriously impair collateral flow and cause more severe perfusion impairment. This mechanism may be only suitable for part of the stroke event. Second, we acquired the images at the interval of 1 hour, and the magic threshold of 4.5 hours for clinical thrombolysis was excluded from acquisition. Some other MR imaging sequences such as PWI, TOF, and SWI were also acquired in 1 session (the acquisition time was nearly 35 minutes in 1 instance), and we were concerned about the quality of the DWI and FLAIR images influenced by the contrast; therefore, we believe that the time point of 4.5 hours should be included in future studies. More animal studies are needed to confirm the reproducibility of our study.
Conclusions
From our study, we found that there was significant correlation between the rFLAIR and the onset time of acute stroke. The rFLAIR might be helpful to predict the onset time of ischemic events. Meanwhile, a new parameter, the degree of DWI-FLAIR mismatch proposed in our study, could be a better indicator for identifying hyperacute ischemic stroke than was the previous visual DWI-FLAIR mismatch.
Acknowledgments
We thank Zhan Zhang and Jun-cheng Dai (Nanjing Medical University, Nanjing, China) for their help in statistics and Zhan-long Ma (Nanjing Medical University, Nanjing, China) for discussion.
Footnotes
Xiao-quan Xu and Qing-quan Zu contributed equally to this work.
This research was supported by National Natural Science Foundation of China (81000653 to S. Liu; 30870710 to H.B. Shi).
Indicates open access to non-subscribers at www.ajnr.org
References
- Received April 20, 2013.
- Accepted after revision May 23, 2013.
- © 2014 by American Journal of Neuroradiology