Abstract
BACKGROUND AND PURPOSE: Asymmetric hypointensity of cerebral veins on susceptibility-weighted imaging has been shown to indirectly reflect tissue hypoxia after cerebral ischemia. We therefore investigated whether patients with prominent asymmetry of the cerebral veins on SWI and a relatively small diffusion-weighted imaging lesion (SWI-DWI mismatch), representing the presence of salvageable tissue, were more likely to benefit from thrombolytic therapy.
MATERIALS AND METHODS: We conducted a retrospective study of the anterior circulation of patients with ischemic stroke with SWI/DWI acquired before thrombolysis. The asymmetry index was defined as the ratio of cerebral vein voxel count between the ischemic and normal hemisphere on the SWI phase map. We defined SWI-DWI mismatch as an asymmetry index score of ≥1.75 with a DWI lesion volume of ≤25 mL. Favorable outcome was defined as modified Rankin Scale 0–2 at 3 months. Univariate and multivariate logistic regression analyses were used to examine the association between the mismatch profile and favorable outcome.
RESULTS: Fifty-four patients undergoing thrombolytic treatment were enrolled in this study. The rate of favorable outcome was significantly higher among patients with baseline SWI-DWI mismatch compared with those without (78% versus 44%; adjusted odds ratio, 6.317; 95% CI, 1.12–35.80; P = .037). Patients with SWI-DWI mismatch were also more likely to have a favorable outcome from reperfusion (91% versus 43%, P = .033) or recanalization (100% versus 40%, P = .013). The accuracy of SWI-DWI mismatch for predicting favorable outcome was higher than that of perfusion-diffusion mismatch (63% versus 48.1%).
CONCLUSIONS: The presence of SWI-DWI mismatch may identify patients with ischemia who would benefit from early reperfusion therapy.
ABBREVIATIONS:
- AI
- asymmetry index
- TIMI
- Thrombolysis in Myocardial Infarction
- Tmax
- time-to-peak of the residue function
Intravenous thrombolysis with recombinant tissue plasminogen activator is a proved treatment for acute ischemic stroke within 4.5 hours of symptom onset.1 However, increasing evidence indicates that it may be more logical to move from this time-based method to a tissue-based imaging paradigm for patient selection for thrombolytic therapy because carefully selected patients may still benefit from thrombolytic therapy beyond 4.5 hours.2 The perfusion-diffusion mismatch model, based on a mismatch between the lesion volume on MR perfusion imaging and diffusion-weighted imaging, has been proposed as a method of selecting patients for reperfusion therapy. Despite theoretic evidence, there is no consensus about the optimal perfusion parameter to accurately define ischemic tissue, and it is uncertain whether perfusion lesion volumes overestimate the penumbra tissue.3,4 Positron-emission tomography is considered the criterion standard for detection of reversibly damaged tissue. However, its availability is limited to a few centers and restricted by complex logistics.5 Thus, until now, the optimal method to identify eligible candidates for reperfusion therapy in the acute setting remains elusive.
More recently, susceptibility-weighted imaging, which was originally called blood oxygen level–dependent venographic imaging, has demonstrated advantages over conventional gradient-echo T2*-weighted imaging in the detection of hemorrhagic events due to its exquisite sensitivity to paramagnetic substances such as deoxyhemoglobin.6 SWI venography allows clear visualization of cerebral veins.7 Shortly after arterial occlusion in patients with acute stroke, there is an increase in deoxyhemoglobin and a decrease in oxyhemoglobin within cerebral veins and tissue capillaries, due to an increase in the oxygen extraction fraction.8⇓–10 This intravascular deoxygenation leads to a signal drop along the course of cerebral veins on SWI venography.11⇓⇓–14 SWI venography may thus provide the oxygen metabolic information about ischemic brain tissue by the noninvasive estimation of blood oxygen level–dependent levels.
A previous study by using gradient-echo T2*-weighted imaging demonstrated that abnormal hypointensity of the deep cerebral veins was correlated with a delayed mean transit time and a large perfusion defect.9 Abnormal hypointensity of superficial cerebral veins also correlates with increased cerebral blood volume.15 Due to the relatively poor resolution of gradient-echo T2*-weighted imaging, no previous studies combined the deep and superficial venous systems as a whole when examining the clinical implications of hypointense cerebral veins. The higher resolution of SWI allowed us to evaluate the ischemic state of the whole brain and the level of tissue hypoxia caused by hypoperfusion. We then developed a novel mismatch model, based on prominent hypointense cerebral veins on SWI and a relatively small DWI lesion (SWI-DWI mismatch), and tested whether this model was more likely to benefit from thrombolytic therapy.
Materials and Methods
Patients
We retrospectively reviewed our prospectively collected data base for consecutive patients with acute ischemic stroke who received intravenous rtPA (alteplase, 0.9 mg/kg, up to a maximum of 90 mg; 10% of the total dosage as a bolus and the rest during 1 hour) within 6 hours after symptom onset from August 2009 to December 2012. Our predefined inclusion criteria for MR imaging–guided intravenous thrombolysis included the following: 1) age older than 18 years; 2) stroke symptoms lasting >1 hour; 3) an NIHSS score of ≥4 or NIHSS score of <4 but with aphasia or severe dysarthria; 4) the absence of intracranial hemorrhage confirmed by SWI; 5) onset-to-treatment time within 4.5 hours and fulfilling standard clinical criteria, or onset-to-treatment time of 4.5–6 hours with large-vessel occlusion on MR angiography and fulfilling the perfusion-diffusion mismatch criteria: DWI lesion of ≤70 mL, a perfusion lesion to diffusion lesion volume ratio of ≥1.2, and an absolute difference between perfusion and diffusion lesion volume of >10 mL.16 Standard IV thrombolysis exclusion criteria were applied according to current guidelines.17 We prospectively obtained baseline demographic and clinical information. All subjects gave written informed consent before the study, and the protocols were approved by the human ethics committee of the hospital. All procedures were conducted according to the principles expressed in the Declaration of Helsinki.
MR Imaging Protocol
Multimodal MR imaging was performed on a 3T system (Signa Excite HD; GE Healthcare, Milwaukee, Wisconsin) before initiation of thrombolysis. Structural imaging included DWI (TR = 4000 ms, TE = 69.3 ms, b-value = 1000 s/mm2, acquisition matrix = 160 × 160, FOV = 240 mm, section thickness = 5 mm, section gap = 1 mm, duration = 32 seconds), a perfusion-weighted sequence (TR/TE = 1500/30 ms, acquisition matrix = 128 × 128, dynamic scans = 50, FOV = 240 mm, section thickness = 5 mm, section gap = 1 mm, gadolinium dose = 15 mL, contrast speed = 4–5 mL/s, duration = l minute 15 seconds), time-of-flight MR angiography (TR/TE/flip angle = 20 ms/3.2 ms/15°, acquisition matrix = 320 × 224, section thickness = 1.4 mm, 3 slabs, duration = 3 minutes 46 seconds), and SWI (3D multiecho T2*-weighted gradient-echo sequence, TR/TE/flip angle = 58 ms/5.0 ms/20°, FOV = 240 mm, acquisition matrix = 384 × 32, section thickness = 2 mm, duration = 3 minutes 27 seconds). The entire duration of the MR imaging protocol was 15 minutes.
Follow-Up Protocol and Clinical Assessments
All patients experiencing thrombolysis underwent follow-up multimodal MR imaging within 24 hours after treatment. Clinical follow-up assessments included the NIHSS score at 1, 4, and 24 hours, 7 days, and 1 month and modified Rankin Scale scores at 1 and 3 months. Early NIHSS improvement was defined as an increase on the NIHSS of at least 8 points between baseline and 1 week after onset or an NIHSS score = 0 at 1 week.18 Favorable outcome was defined as mRS 0–2 at 3 months.
Radiologic Assessment
DWI lesion volumes were measured by using a semiautomated thresholding algorithm that identified the region with intensity higher than that in the contralateral frontal lobe by >3 SDs.19 Artifactual lesion areas were visually identified by an experienced neuroradiologist (J.S.) and were manually removed. Time-to-peak of the residue function (Tmax) maps were generated by deconvolution of the tissue concentration–time curve by using an arterial input function from the contralateral middle cerebral artery.19,20 The perfusion lesion was defined as a Tmax delay of >6 seconds.21 Reperfusion was defined as a ≥50% and ≥10 mL reduction of PWI lesion volume22 between baseline and follow-up within 24 hours of MR imaging. PWI lesions of ≤3 mL were excluded.23
The MRA was rated independently by 3 experienced stroke neurologists (M.L, Z.C., and J.S.), who then reached consensus by using the Thrombolysis in Myocardial Infarction (TIMI) grading scale16,24: 0 = complete occlusion, which was defined as lack of flow signal of a vascular segment and distal vessels; 1 = severe stenosis, defined as severe or critical stenosis of a vascular segment with significant reduction of flow signal distal to stenosis; 2 = mild-to-moderate stenosis, which was characterized by stenosis with normal distal flow signal; 3 = normal arterial caliber. Recanalization was defined as improvement of TIMI grading by ≥2 points from baseline to follow-up MRA.
Definition of the Asymmetry Index
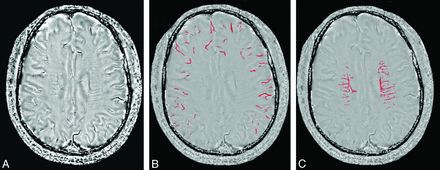
We used a high-pass filter with a central matrix size of 32 × 32 to remove background field inhomogeneities to create the corrected SWI phase images. We used MRIcro software (http://www.mccauslandcenter.sc.edu/mricro/mricro/mricro.html) to assess venous structures in 5 consecutive sections around the level of the lateral ventricles (from immediately above the basal ganglia to the highest section including the ventricle) because these sections include most of the cerebral veins draining the anterior circulation territory. The segmentation threshold for deep and superficial cerebral veins was determined as 2 SDs in voxel intensity below the mean of the fitted distribution of white matter and cortex, respectively (Fig 1). Bilateral venous voxel counts were computed, and an asymmetry index (AI) was defined as the ratio of the venous voxel number between the ischemic and normal hemispheres to eliminate individual bias.25⇓–27
Image postprocessing and segmentation of cerebral venous structures based on SWI phase images. The SWI phase image (A) was used to calculate the number of voxels of superficial cerebral veins (B) and deep cerebral veins (C) for quantification after segmentation. The asymmetry index of cerebral veins was set as a ratio of voxel numbers of cerebral veins between the ipsilateral and contralateral side.
Definition of SWI-DWI Mismatch
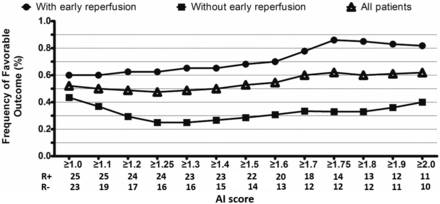
The interaction among early reperfusion, AI score, and favorable outcome was assessed (Fig 2), and receiver operating characteristic analysis was used to determine the cutoff value for an optimal AI score when it gave the highest Youden index.20 We defined SWI-DWI mismatch as an AI score greater than the optimal threshold and a DWI lesion of ≤25 mL.28,29 To examine whether patients with SWI-DWI mismatch were more likely to benefit from reperfusion or recanalization after rtPA therapy, we compared the rate of favorable outcome from reperfusion or recanalization between patients with and without SWI-DWI mismatch. We also compared the accuracy of this novel SWI-DWI mismatch with the perfusion-diffusion mismatch. We used several different perfusion-diffusion mismatch definitions: 1) standard PWI-DWI mismatch: mismatch ratio of ≥1.2 and absolute mismatch of ≥10 mL16; 2) optimal PWI-DWI mismatch: mismatch ratio of ≥2.6 and absolute mismatch of ≥10 mL20; 3) target PWI-DWI mismatch: mismatch ratio ≥ 1.2, absolute mismatch ≥ 10 mL, Tmax ≥ 8 seconds, lesion ≤ 100 mL, and DWI ≤ 100 mL.30
The AI score was positively correlated with the frequency of favorable outcome in patients with early reperfusion. The optimal AI score was set as 1.75 based on maximizing the Youden index (sensitivity, 83%; specificity, 67%; Youden index, 53%) for predicting favorable outcome in patients with early reperfusion. R (+) indicates the number of patients with early reperfusion; R (−), the number of patients without early reperfusion.
Statistical Analysis
We divided patients into high and low AI groups, by using the Youden-derived threshold. We also compared variables between patients with and without SWI-DWI mismatch. We used the Fisher exact test to compare dichotomous variables between groups, the t test for normally distributed continuous variables, and the Wilcoxon rank sum test for ordinal variables and non-normally distributed continuous variables. Univariate and multivariate logistic regression analyses were used to investigate whether SWI-DWI mismatch was an independent predictor of favorable outcome. Statistical significance was set at P < .05. All statistical analyses were performed by using SPSS, Version 19 (IBM, Armonk, New York).
Results
Eighty consecutive patients had multimodal MR imaging, and 60 had cerebral infarction in the anterior circulation territory. Six patients were excluded before the analysis because of poor-quality SWI due to dental artifacts (1 patient) and motion artifacts (5 patients), which interfered with the assessment of cerebral veins. Thus 54 patients were included in the analysis. The mean age of the patients was 69 ± 13 years, and the median baseline NIHSS score was 12 (interquartile range, 5–17). Eight patients received thrombolytic therapy beyond 4.5 hours according to the criteria described in “Materials and Methods.” Favorable outcome occurred in 3/3 with SWI-DWI mismatch versus 1/5 (20%) without SWI-DWI mismatch.
Optimal AI Score for SWI-DWI Mismatch Analysis
As shown in Fig 2, in the presence of reperfusion, the rate of favorable outcome correlated with increasing AI score. Receiver operating characteristic analysis revealed that the optimal threshold for the AI score was 1.75 (sensitivity, 86%; specificity, 67%; Youden index, 53%) for predicting favorable outcome from early reperfusion.
AI Score and Hypoperfusion Lesion Volume
Table 1 summarizes the baseline and 24-hour follow-up variables in patients with high (n = 27) versus low AI scores (n = 27). Univariate analysis showed that patients with high AI scores had more frequent arterial occlusion (median TIMI 1 [interquartile range, 0–1] versus 2 [interquartile range, 0–3], P = .002) and larger perfusion lesion volume (120 ± 83 mL versus 58 ± 67 mL, P = .004) than those with a low AI score. Figure 3 illustrates the relationship among AI score, perfusion lesion volume, and TIMI grading.
Univariate analysis of the baseline variables according to the asymmetry index of cerebral veinsa
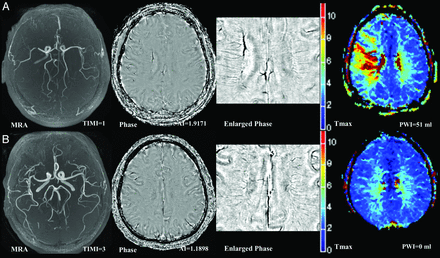
Representative pretreatment MR imaging in patients receiving thrombolysis. The first column on the left depicts time-of-flight MR angiography; the second and third columns depict phase images of susceptibility-weighted imaging and an enlarged view focusing on the deep cerebral veins. The right column depicts Tmax maps of perfusion-weighted MR imaging. Patients with a high AI score (A, TIMI = 1, AI score = 1.92, PWI lesion volume = 51 mL) had a lower TIMI score and larger PWI lesion volume than those with low AI scores (B, TIMI = 3, AI score = 1.19, PWI lesion volume = 0 mL).
SWI-DWI Mismatch and Favorable Outcome
SWI-DWI mismatch, defined by a high AI score (AI ≥ 1.75) with a small DWI lesion volume (≤25 mL), was present in 18 of 54 patients (33%). Univariate analysis showed that patients with SWI-DWI mismatch were more likely to have early NIHSS improvement (67% versus 31%, P = .019) and favorable outcome (On-line Table 1) compared with those without SWI-DWI mismatch (78% versus 44%, P = .024). The rates of reperfusion, recanalization, and hemorrhagic transformation were not significantly different between these 2 groups. Logistic regression analysis revealed that the presence of SWI-DWI mismatch (OR = 6.317; 95% CI, 1.12–35.80; P = .037) was independently associated with favorable outcome (On-line Table 2).
Favorable Outcome of SWI-DWI Mismatch based on Reperfusion
Table 2 shows that 11 patients (61%) with SWI-DWI mismatch and 14 (39%) patients without it achieved reperfusion. Patients with SWI-DWI mismatch were more likely to obtain favorable outcome from reperfusion than those without SWI-DWI mismatch (91% versus 43%, P = .033).
Different outcomes in patients with or without SWI-DWI mismatch based on reperfusion
Comparison of Accuracy between SWI-DWI Mismatch and PWI-DWI Mismatch
As shown in Table 3, the accuracy of SWI-DWI mismatch (63.0%) was higher than that of PWI-DWI mismatch (37.0% for standard PWI-DWI mismatch, 44.4% for optimal PWI-DWI mismatch, 48.1% for target PWI-DWI mismatch).
The sensitivity, specificity, and accuracy between SWI-DWI and PWI-DWI mismatchesa
Discussion
Our study proposes a novel SWI-DWI mismatch paradigm for predicting neurologic outcome after intravenous thrombolysis. Patients with SWI-DWI mismatch had a higher rate of favorable outcome than those without this mismatch (78% versus 44%). The accuracy of SWI-DWI mismatch was higher than that of PWI-DWI mismatch in the current study. Thus, SWI-DWI mismatch may provide an alternative method for selecting patients who are good candidates for thrombolytic therapy.
Deoxyhemoglobin is used as an endogenous contrast agent due to its paramagnetic properties on SWI. Because venous blood volume contains higher concentrations of deoxyhemoglobin, the signal change on SWI is mainly related to venous structures.11⇓⇓–14 In the setting of acute arterial occlusion, decreased oxygen supply causes an increased tissue oxygen extraction fraction, which leads to the increased level of deoxyhemoglobin and results in signal reduction of ipsilateral cerebral veins in the ischemic tissue on phase maps.8⇓–10 Thus, as in the previous studies on gradient-echo T2*-weighted imaging,25,26 we used asymmetry of cerebral veins to reflect the change of oxygen extraction fraction in the ischemic hemisphere.
In the current study, we found that patients with a high cerebral vein AI score after ischemic stroke were more likely to have severe arterial occlusion and hypoperfusion. This outcome is consistent with that in a previous study which, by using gradient-echo T2*-weighted imaging, demonstrated that abnormal prominent deep cerebral veins were correlated with a delayed MTT, a large perfusion defect, and increased CBV.9 Prominent superficial cerebral veins have also been correlated with an increase in CBV.15 Recently, an Alberta Stroke Program Early CT Score–based rating of asymmetric prominent vessels on SWI was shown to correlate best with MTT in patients with nonlacunar ischemic stroke within 24 hours after symptom onset.27 Therefore, extensive hypointense cerebral veins on SWI may be a useful indicator of ipsilateral large-artery occlusion and severe hypoperfusion. Most important, unlike the previous study, which found an association between abnormal visualization of the superficial venous system on gradient-echo T2*-weighted imaging and smaller DWI lesions,15 we did not find such an association between the AI score and DWI lesion volume. According to previous literature,31,32 the superficial cerebral veins and deep cerebral veins differ in anatomic structure and function.25,33 We, therefore, considered it improper to compare the change of partial draining veins with DWI lesions.
We then tested the SWI-DWI mismatch, which may represent the presence of salvageable tissue based on tissue oxygen metabolism, and found that patients with SWI-DWI mismatch were more likely to benefit from thrombolytic therapy. One might argue that the relatively small DWI lesion volume in SWI-DWI mismatch may have a critical impact on patient outcome. However, we did not find a significant relationship between posttreatment neurologic outcome and either baseline DWI lesion volume or the AI score alone from logistic regression. Several studies have demonstrated metabolic derangement and evolution of infarction in brain regions with increased oxygen extraction fraction by PET.34,35 An increased oxygen extraction fraction may be a target for acute therapy to improve compromised cerebral circulation. Our study further revealed that in the presence of reperfusion, patients with SWI-DWI mismatch were more likely to have favorable outcome, compared with patients without it. We speculate that the presence of SWI-DWI mismatch after arterial occlusion in acute stroke may indicate salvageable tissue. The compensatory increase of oxygen uptake by tissue can provide the hypoxic tissue with a chance to revive if there is timely reperfusion. Therefore, we believe this MR imaging profile can identify the subgroup who may benefit from early reperfusion therapy. However, quantitative metabolic parameters that relate directly to the cerebral metabolic rate of oxygen consumption detected by PET are needed to assess this hypothesis.
The main advantage of using the SWI-DWI mismatch is that it provides a new concept to examine the penumbra on the basis of tissue oxygen metabolism. Although the SWI-DWI mismatch is not able to directly measure the cerebral metabolic rate of oxygen consumption, the concept of SWI-DWI mismatch is more closely aligned with the fundamental pathogenesis of neuronal energy disruption in acute ischemia than the perfusion-diffusion mismatch. In the perfusion-diffusion mismatch, identifying a perfusion threshold to reliably differentiate critically hypoperfused tissue21 from benign oligemia, which will survive regardless of reperfusion,20,36 is a major challenge. The site of vascular occlusion and the extent of collateral blood flow also affect the complex relationship between perfusion lesions and their final fate. Another potential advantage of SWI over MR perfusion is that it obviates gadolinium contrast, which is contraindicated in patients with severe renal insufficiency or gadolinium allergy.37 The SWI-DWI mismatch may therefore be a useful alternative method that does not require MR perfusion to select patients who are good candidates for thrombolytic therapy. Moreover, our finding that patients with SWI-DWI mismatch achieved more favorable outcome based on successful reperfusion lends support to its future application in patients treated with an endovascular approach. In the current study, approximately 33% of patients had SWI-DWI mismatch. This was similar to the proportion (36%) with “optimal” mismatch (mismatch ratio of >2.6) in post hoc analysis of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution study.20 These patients may be a suitable “responder” subgroup for randomization between reperfusion therapy and standard therapy.
There are limitations to our study. First, the exploratory nature of this study and the relatively small number of subjects necessitate the validation of the SWI-DWI mismatch model in an external dataset. Our conclusions should be considered hypothesis-generating and require further testing in prospective studies. Second, the asymmetry index of signal reduction along the cerebral veins may vary among different MR imaging magnetic field strengths and can be influenced by the presence of microbleeds and a previous large ischemic stroke. Moreover, the segmentation of cerebral veins may be partly affected by cortex and ependymal epithelium signals. Third, the relationship of oxygen consumption and SWI-DWI mismatch needs further validation by PET studies. Fourth, the postprocessing and calculation of the AI index would take an additional 3 minutes, which may delay thrombolysis. Fifth, the definition of reperfusion and recanalization by MR imaging are still not unified. Last, our conclusions were all based on patients receiving tPA, without a control group, though we analyzed the outcome on the basis of reperfusion. Thus, we could not clarify whether withholding thrombolytic therapy in patients without a mismatch would yield better results. A randomized controlled trial would be needed to validate the usefulness of the SWI-DWI mismatch in the selection of patients for reperfusion therapy.
Conclusions
A high AI score of cerebral veins may be related to arterial occlusion or severe hypoperfusion. The presence of an SWI-DWI mismatch may predict a favorable outcome after intravenous thrombolysis. Our data provide evidence that SWI venography, related to oxygen metabolism, may provide an alternative for the evaluation of patients with acute ischemic stroke.
Acknowledgments
We are grateful for the support from our patients. The authors thank Yi-hong Zhu, MD, from Zhejiang University School of Public Health for the critical verification of statistics, and Bruce Campbell, MD, from the Department of Neurology, Royal Melbourne Hospital, Australia, for the critical reading of the manuscript.
Footnotes
Disclosures: Min Lou—RELATED: grant support: Science Technology Department of Zhejiang Province (grant 2013C13G2010032), Zhejiang Provincial Natural Science Foundation (grant LR12H09001), and the National Natural Science Foundation of China (grant 81171095).* *Money paid to the institution.
This work was supported by the Science Technology Department of Zhejiang Province (grant 2013C13G2010032), Zhejiang Provincial Natural Science Foundation of China (grant LR12H09001), and the National Natural Science Foundation of China (grant 81171095).
Indicates open access to non-subscribers at www.ajnr.org
References
- Received January 17, 2014.
- Accepted after revision March 23, 2014.
- © 2014 by American Journal of Neuroradiology