Abstract
BACKGROUND AND PURPOSE: Optimizing the utilization of surveillance PET/CT in treated HNSCC is an area of ongoing research. Our aim was to determine the negative predictive value of PET/CT in patients with treated head and neck squamous cell cancer and to determine whether negative PET/CT reduces the need for further imaging surveillance.
MATERIALS AND METHODS: We evaluated patients with treated HNSCC who underwent posttreatment surveillance PET/CT. During routine clinical readouts, scans were categorized as having negative, probably negative, probably malignant, or malignant findings. We followed patients clinically and radiographically for at least 12 months from their last PET/CT (mean, 26 months; median, 28 months; range, 12–89 months) to determine recurrence rates. All suspected recurrences underwent biopsy for confirmation.
RESULTS: Five hundred twelve patients (1553 scans) were included in the study. Two hundred fourteen patients had at least 1 PET/CT with negative findings. Of the 214 patients with a scan with negative findings, 19 (9%) eventually experienced recurrence, resulting in a NPV of 91%. In addition, a subgroup of 114 patients with 2 consecutive PET/CT examinations with negative findings within a 6-month period was identified. Only 2 recurrences were found in this group, giving a NPV of 98%.
CONCLUSIONS: In patients treated for HNSCC, a single PET/CT with negative findings carries a NPV of 91%, which is not adequate to defer further radiologic surveillance. Two consecutive PET/CT examinations with negative findings within a 6-month period, however, resulted in a NPV of 98%, which could obviate further radiologic imaging in the absence of clinical signs of recurrence.
ABBREVIATIONS:
- HNSCC
- head and neck squamous cell carcinoma
- NPV
- negative predictive value
- T
- tumor
Squamous cell carcinoma accounts for most of the approximately 50,000 annual new cases of head and neck cancer in the United States.1 A radiologic examination is crucial in staging patients, monitoring response to therapy, and conducting surveillance of treated disease.2 CT, MR imaging, and PET have been used for these evaluations, with combined PET/CT showing greater accuracy than either PET or CT alone.3 PET/CT has an overall 90% sensitivity in localizing recurrent disease and an 86% accuracy for finding residual disease.4⇓⇓–7
Current literature on PET/CT in HNSCC has focused on detection, staging, and monitoring response to therapy, while research in the area of posttreatment surveillance is limited.2 To reduce both medical cost and the radiation dose to patients, identification of an efficient and effective surveillance scheme is critical, but there is no widely accepted protocol for the use of PET/CT in the surveillance of HNSCC, leading to the potential misapplication or overuse of this technique.8 Previous literature has suggested that it is wasteful to perform PET/CT for HNSCC surveillance >24 months after the conclusion of therapy.9 There may be subsets of patients, however, in whom radiologic surveillance can be curtailed even earlier, thus saving money and reducing patient inconvenience without increasing morbidity. In particular, patients with negative PET/CT surveillance scans might be best managed with a reduced frequency of scanning or with a complete cessation of further scans.
The purpose of this study was to determine the negative predictive value of PET/CT in HNSCC and to determine whether a negative PET/CT scan reduces the need for further radiologic surveillance.
Materials and Methods
Patient Selection
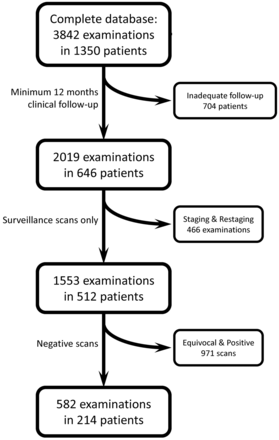
The institutional review board of the University of Pittsburgh Medical Center approved this retrospective study. The records of all patients who had biopsy-proved HNSCC who underwent PET/CT examinations at the University of Pittsburgh Medical Center between 2002 and 2010 were included. Medical records were extracted from a dedicated Head and Neck Oncologic Data Repository. All nonsquamous cell malignancies of the head and neck were excluded from this study. The resulting population numbered 1350 patients with 3842 PET/CT scans. Patients were required to have had at least 12 months of clinical and radiographic follow-up at our institution from the time of their last PET/CT to be included in the study. This applied to 646 patients with 2019 PET/CT scans. Of the 2019 scans, 1553 scans (77%) from 512 patients were obtained for the purpose of surveillance of treated HNSCC. Scans that were obtained for the purpose of staging or restaging were excluded from the study. Of these 512 patients, a subset of 214 patients (42%) who had negative PET/CT findings at any point during their surveillance was identified. These 214 patients had at least 12 months of follow-up after their PET/CT examinations with negative findings. Second primary malignancies that were discovered during the study period were not considered to indicate false-negative PET/CT findings, and these patients remained in the study for the evaluation of recurrence of their first cancer (Fig 1).
Data flow chart of patient selection.
Surveillance Protocol
Clinical surveillance with physical examination and endoscopy was performed every 6–8 weeks for the first year after the conclusion of therapy. Surveillance PET/CT was performed at 3-month intervals, starting 2 months after the conclusion of therapy. Surveillance scans were routinely obtained at 2, 5, 8, and 14 months after therapy, as per our institutional protocol. Scans were obtained at 11 months only if there were findings on the 8-month scan that required follow-up. Patients who developed suspicious clinical findings were immediately scanned with PET/CT, but these examinations were not included in this cohort (Fig 1). The patient's original examination with negative findings was still included in the cohort, however, so that patients with clinically detected recurrence would be classified as having false-negative PET/CT findings. Additional radiologic surveillance was performed only in patients who had findings that merited radiologic follow-up on the 14-month scan. Patients with suspicious radiologic or clinical findings underwent biopsy for confirmation or exclusion of recurrence, and patients with a biopsy negative for recurrence returned to the surveillance regimen.
Not all patients treated for HNSCC at our institution are entered into this intense radiologic imaging surveillance regimen. Very low-risk patients are not usually followed by PET/CT, and entrance into this imaging surveillance protocol is at the discretion of the referring clinical services. Patients with small tumors in anatomically sensitive areas, such as the oral tongue or supraglottic larynx, which might predispose to metastatic disease are often followed with this imaging surveillance regimen.
Imaging Parameters
Patients fasted for at least 6 hours before the FDG-PET/CT examination with the exception of water intake. IV access was established for blood glucose testing and subsequent radiopharmaceutical and iodinated contrast administration. If serum glucose levels were >200 mg/dL, the examination was rescheduled. Each patient received 10–17 mCi of IV [18F] FDG. After administration of the radiopharmaceutical, the patients rested quietly during the 60-minute uptake period.
The studies were performed on 1 of 7 PET/CT scanners with 2–64 detector rows (Discovery; GE Healthcare, Milwaukee, Wisconsin; and Emotion; Siemens, Erlangen, Germany). The first 1016 scans were obtained on 2- and 4-section PET/CT scanners no longer in operation, and the next 2826 scans were obtained on 16- to 64-section PET/CT scanners presently in operation. The CT scan parameters were 120–130 kV(peak), variable/smart milliampere, and 3.75-mm collimation. CT scanning commenced following a 30-second delay after the administration of IV contrast (125-mL iopamidol, Isovue-370; Bracco Diagnostics, Princeton, New Jersey) and was performed from the top of the skull through the abdomen. After the CT, PET data were acquired by using a 4-minute bed position. The PET acquisition included a dead-time correction and on-line delayed coincidence subtraction to correct for random coincidences. The helical CT scan was reconstructed by filtered back-projection into 512 × 512 pixel images with a section thickness of 2.4 mm to match the PET scan. Images of the neck were reconstructed with a small FOV to improve visualization of the primary tumor site and regional nodes. Images were reconstructed by using ordered subset expectation maximization with 2 full iterations of 8 subsets. Rescaled CT images were used to produce attenuation-correction values for the PET emission reconstruction.
Image Analysis
All PET/CT examinations were interpreted as part of the normal clinical workflow by 1 of 2 certificate of added qualification–certified neuroradiologists with 8 and 10 years of dedicated head and neck imaging experience. Findings of each examination were classified as follows at the time of initial clinical interpretation: 1) negative examination (no evidence of malignancy), 2) probably negative (CT or PET findings that are most likely due to inflammation, treatment effects, or altered physiology), 3) probably malignant (findings requiring biopsy for confirmation of disease), or 4) malignant (unequivocal disease progression). This determination was based on the overall impression of the radiologist of both PET and CT data; standardized uptake value thresholds were not applied. Patients with FDG uptake that was definitively physiologic, such as in muscle or brown fat, were considered as having “negative” findings. This categorization scheme is an integral part of our usual clinical practice, and only the routine clinical interpretations were used for this study.
Results
There was a total of 3842 examinations in the complete data base. Of these, 1347 (35%) were interpreted as having negative findings.
After application of exclusion criteria to the 3842 examinations (only patients with HNSCC, adequate follow-up, and scans obtained for surveillance purposes), 1553 scans in 512 patients remained in the study (Fig 1). Table 1 summarizes the demographic and staging information for these patients. Table 2 further stratifies these patients by site of tumor origin and T stage. All patients underwent at least 12 months of clinical and radiologic follow-up from their last PET/CT (mean follow-up, 26 months; median, 28 months; range, 12–89 months).
Patient demographics, TNM stages, and therapya
Primary site of origin and tumor stagea
Of the 1553 scans included in the study, there were 582 scans (37%) in 214 patients that were interpreted as having negative findings. Table 3 summarizes the T stage and site of origin for this subset of 214 patients with at least 1 PET/CT examination with negative findings. Of the 214 patients with scans with negative findings, 19 (9%) eventually recurred. Of the 19 recurrences, 11 (58%) were local recurrences, 6 (32%) were regional recurrences, and 2 (10%) were distant metastases. Table 4 provides additional clinical information regarding the 19 patients who experienced recurrence despite a prior PET/CT with negative findings. Nineteen recurrences in 214 patients resulted in a negative predictive value for surveillance PET/CT of 91%.
Primary site of origin and tumor stage for the subset of 214 patients with negative PET/CT findingsa
Clinical Information of the 19 patients with negative PET/CT findings who had a recurrence
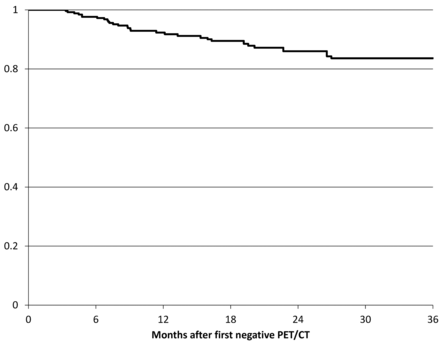
The disease-free survival curve of patients with negative surveillance PET/CT findings (Fig 2) shows that the first recurrences are detected around 3 months after the PET/CT with negative findings. Few recurrences are detected after 30 months.
Disease-free survival curve of patients with negative findings on PET-CT surveillance examinations after treatment for HNSCC. Time zero was chosen as the date of first PET/CT examination with negative findings.
Of the group of 214 patients with negative PET/CT findings, 114 (53%) had 2 consecutive PET/CT scans with negative findings within 6 months. The other 100 patients did not go on to have a second PET/CT examination with negative findings within 6 months because of the following: 17 patients had recurrence; 37 patients were tumor-free and had no further scans obtained; 21 patients did have a subsequent PET/CT examination with negative findings, but it was >6 months after the prior examination with negative findings and thus did not meet our criteria; and 25 patients had subsequent non-negative findings on PET/CT examinations but never actually had a recurrence. Among the 114 patients with 2 consecutive scans with negative findings, there were only 2 recurrences, 1 occurring at 9 months and the other at 37 months. Thus, 2 consecutive PET/CT scans with negative findings within 6 months of each other provide a negative predictive value of 98%.
Discussion
This study suggests that radiologic surveillance can be terminated early in a subset of patients with treated HNSCC who have 2 consecutive PET/CT scans with negative findings within 6 months of each other, a conclusion that has important economic implications for the use of expensive health care resources. Earlier studies have suggested that the use of PET/CT beyond 24 months after therapy may be wasteful,9 and the current work provides further data to help reduce overuse of PET/CT in this clinical setting. Of the 214 patients in this study with a single PET/CT scan with negative findings, there were 19 recurrences, giving a negative predictive value of 91%. In the subset of 114 patients with 2 consecutive PET/CT scans with negative findings within 6 months of each other, there were only 2 recurrences, improving the negative predictive value to 98%. Of those 2 recurrences, 1 occurred at 9 months after the first PET/CT scan with negative findings, and the other, at 37 months after the first PET/CT scan with negative findings. The second recurrence at 37 months would be beyond the window of most radiologic surveillance protocols. These results suggest that, in patients with 2 consecutive PET/CT scans with negative findings, there is a <1% recurrence rate within the generally accepted radiologic surveillance window.
Prior studies with fewer patients have shown similar results, with negative predictive values as high as 97%–100%.10⇓–12 Yao et al10 examined 85 patients who had HNSCC treated with intensity-modulated radiation therapy and had subsequent FDG PET scans in follow-up. Initial scans had negative findings in 64 patients, and of these patients, there was only 1 local recurrence 7 months after the initial study. In a study to compare MR imaging/CT with PET alone, Kubota et al13 studied a group of 36 patients and found a NPV for PET of 91%.
HNSCC recurs at rates as high as 50% in locally advanced disease. Regular posttreatment follow-up allows earlier detection of disease recurrence and initiation of additional therapies such as surgery or re-irradiation.12,14,15 The National Comprehensive Cancer Network currently only recommends imaging within 6 months of treatment for certain types of head and neck cancers at specific stages, and the type of imaging is not specified.8 On the basis of the results of this study, we would recommend more specific guidelines that include a second PET/CT examination performed within 6 months of the first scan so that radiologic surveillance can be confidently abridged.
Combined PET/CT has been shown to be a superior to PET or CT alone for surveillance of HNSCC3; however, the optimal frequency and duration of surveillance imaging is not well-studied. Greven et al16 showed a high false-negative rate for a PET scan obtained 1 month after treatment but a much greater accuracy when scans were obtained 4 months after treatment; specifically, 0 of 18 patients had a recurrence after negative findings on a scan at 4 months. Other studies have shown similar data supporting the initial use of PET/CT scans 3–5 months after completion of treatment. Our results, as well as those in previous studies,9 suggest that additional PET/CT scans are of value in surveillance protocols.
Given our results of a NPV of 91% for 1 scan, it is probably not reasonable to stop radiologic surveillance after 1 scan with negative findings. With an NPV of 98% for 2 consecutive scans, however, it may be practical to then follow patients with clinical surveillance alone. Scanning beyond 24 months after therapy is likely not of value in this setting.9
Regarding scan frequency, we detected recurrences beginning at 3 months after a PET/CT scan with negative findings. Scans obtained earlier may miss recurrent disease and scans obtained later may lead to a delay in treatment of recurrence. The timing of the first post-therapy surveillance scan remains controversial. Most institutions perform radiologic examinations between 8 and 12 weeks after the conclusion of therapy, but no definite consensus exists.
In calculating negative predictive values, a high NPV can be obtained by interpreting very few examinations as negative. At our institution, however, 35% of all findings in head and neck cancer PET/CT cases in the dedicated Head and Neck Oncologic Data Repository were interpreted as negative, which indicates that a high NPV can be achieved without sacrificing specificity. The rate of negative interpretation among patients included in the study was similar (37%), suggesting that our selection criteria did not bias us toward or away from examinations with negative findings. Scan findings interpreted as “probably negative” (increased FDG uptake that is likely physiologic or inflammatory, or incomplete response to therapy) are not considered the same as “negative.” Our results apply only to scans with truly negative findings; patients with less definitive results merit a more aggressive surveillance regimen. The optimal imaging protocol for such patients is an area for future research.
There are several limitations to this study. First, this was a single-center retrospective study, and the results and recommendations have not been validated prospectively. Second, the results rely on particular PET/CT protocols for head and neck cancer, including the use of intravenous contrast as well as experienced readers, and we strongly support the use of intravenous contrast and small-FOV neck images to improve the accuracy of PET/CT.17 We also believe that PET/CT is best interpreted as a combined technique, with both components holding equal weight during interpretation.2 Additionally, our findings are also dependent on the accuracy of a large clinical data repository. We did not attempt to analyze a subsets of patients on the basis of human papillomavirus status or therapeutic modalities; this is an area for further study. An intentional limitation of this study is the evaluation of only NPV. This was done to present a singular clinically relevant message regarding optimal imaging surveillance of a patient who has been treated for head and neck cancer. Finally, the most important goal of cancer surveillance is to identify recurrences with a potential for cure. We did not attempt to determine whether the recurrences in our patient cohort were treatable or whether identifying these recurrences prolonged overall survival.
Conclusions
In patients with treated HNSCC, a single PET/CT scan with negative findings obtained at any time after the conclusion of therapy has a NPV of 91%. There are a sufficient number of subsequent recurrences, however, to warrant a second PET/CT scan obtained 6 months after the scan with negative findings. If this second scan also has negative findings, then the NPV rises to 98%, which is sufficient to suspend radiologic surveillance in asymptomatic patients.
Acknowledgments
We thank Arpita Mehta, Nasir Siddiqui, Vineet Khanna, Daniel Beswick, and Jonathan Yu for their assistance in adding PET/CT data to the Head and Neck Oncology Data Repository.
Footnotes
Disclosures: Gregory J. Kubicek—UNRELATED: Other: American College of Radiation Oncology (ACRO), Comments: chart review for ACRO (less than $1000). Umamaheswar Duvvuri—UNRELATED: Consultancy: Intuitive Surgical, Comments: I am a national proctor for da Vinci robotic surgery, Grants/Grants Pending: Medrobotics Inc.* *Money paid to the institution.
REFERENCES
- Received November 28, 2011.
- Accepted after revision October 29, 2012.
- © 2013 by American Journal of Neuroradiology