Abstract
SUMMARY: While uncommon, CNS-IRIS developing after the initiation of HAART in the setting of HIV-related severe immunosuppression is characterized by an intense inflammatory reaction to dead or latent organisms or to self-antigens due to a heightened but dysregulated immune response. While this reaction can range from mild to fulminating, encompassing a very wide clinical spectrum, it is important to recognize because changes in medical management may be necessary to prevent neurologic decline and even death. Once contained, however, this inflammatory response can be associated with improved patient outcome as immune function is restored. Among the infectious organisms that are most commonly associated with CNS-IRIS are the JC virus and Cryptococcus organisms, which will be the subject of this review. CD8 cell infiltration in the leptomeninges, perivascular spaces, blood vessels, and even parenchyma seems to be the pathologic hallmark of CNS-IRIS. While recognition of CNS-IRIS may be difficult, the onset of new or progressive clinical symptoms, despite medical therapy and despite improved laboratory data, and the appearance on neuroimaging studies of contrast enhancement, interstitial edema, mass effect, and restricted diffusion in infections not typically characterized by these findings in the untreated HIV-infected patient should raise the strong suspicion for CNS-IRIS. While CNS-IRIS is a diagnosis of exclusion, the neuroradiologist can play a critical role in alerting the clinician to the possibility of this syndrome.
ABBREVIATIONS:
- ART
- antiretroviral therapy
- CM
- cryptococcal meningitis
- HAART
- highly active antiretroviral therapy
- IRIS
- immune reconstitution inflammatory syndrome
- JCV
- JC virus
- OI
- opportunistic infection
- PML
- progressive multifocal leukoencephalopathy
- Th
- T-helper cell
IRIS, first described in 1992, occurs most commonly in the setting of HIV immunosuppression,1 the focus of this article. When IRIS occurs in HIV-infected individuals, it develops within weeks, months, or, rarely, years after the initiation of HAART and represents an exuberant inflammatory response to an antigen that is either to a dead or dying organism resulting from an OI or a viable pathogen from a persistent infection, or to self-antigens.1⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–46 This exaggerated inflammatory response can be recognized by the development of new clinical symptoms or worsening of existing clinical symptoms despite adequate treatment of the OI and by specific abnormalities on MR imaging or CT that are usually distinct from the imaging findings that are characteristic of that particular offending OI.1⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–46 While this robust inflammatory response is usually self-limiting and often associated with mild symptoms and eventual immune restoration, it can be fulminating, with death ensuing a short time after symptom onset.1 Furthermore, because IRIS has been reported by many investigators to have an overall incidence at least as high as 25%–35%, increasing to 45% in those with underlying OIs,2,9,10,16 it significantly negatively impacts the HIV-infected population on HAART by increasing the number of procedures, number of hospitalizations, and the overall morbidity in this patient cohort.2
Morbidity and mortality rates are even more exaggerated in developing countries, indicating a global health concern.1 It is evident, then, that strategies for promptly recognizing and treating patients with IRIS are critical to the ongoing fight against HIV infection in the post-HAART era so that further strides in improving quality of life can be ensured. Also critical is the realization that IRIS might be averted if steps are taken to prevent CD4 counts from dropping below 50 cells per microliter and OI is prevented.5
In those HIV-infected patients on HAART who do develop IRIS when their T-cell antigen-specific immunity is reconstituted following an anergic state,8 the risk factors for the development of IRIS include the following: 1) the patient being HAART-naïve, which allows a more intense inflammatory response to develop2,7,8; 2) the patient being severely immunocompromised with very low CD4 counts (<50 cells per cubic millimeter) at the initiation of ART5,7,11; 3) high pre-HAART HIV-1 RNA levels; 4) falling HIV-1 RNA levels in response to HAART initiation, especially when this fall occurs rapidly and results in significant level reductions and when it takes place within 90 days of the introduction of HAART2,8,12⇓–14; 5) rising CD4 counts after initiation of HAART, especially later in the course of therapy after falling HIV-1 RNA levels have resulted in an initial redistribution of memory CD4 lymphocytes2,8; 6) OI or the patient on treatment for OI when HAART is initiated, especially within a month of the OI diagnosis, because the increased antigenic burden evokes a more robust inflammatory response2,7,16; 7) resumption of HAART after an interruption; 8) younger age; 9) male sex; and 10) genetic factors that alter the clearance of the pathogen (such as with herpesviruses or mycobacteria) or enhance the immune response to it via polymorphisms in cytokine genes.8,15
While some of these risk factors are still being debated, such as age and sex,8 and while criteria are still being expanded and further defined2,17 and the pathogenesis of IRIS remains not well-understood (with some investigators suggesting that there may even be different mechanisms for different pathogens),7,18,28 there is general acceptance that IRIS can be diagnosed in an HIV-infected individual when there is evidence that the patient's immune system is reconstituting (higher CD4 counts and decreasing HIV-1 RNA levels), yet the patient is paradoxically worsening with the development of new symptoms that cannot be explained by drug toxicity, OI, medical noncompliance, or allergic reactions.6,16,19 IRIS then is often a diagnosis of exclusion.8 Diagnosis, however, can be supported by the detection of atypical imaging and laboratory findings, such as new imaging patterns and laboratory tests that might not show viable organisms. Pathologically, T-cell infiltration confirms the diagnosis.1 Conversely, a factor that does not seem to alter the development of IRIS includes the specific type of HAART.2 For example, patients on protease-containing regimens had a similar risk of the development of IRIS as those HIV-infected individuals on non-protease-containing regimens.2
Curiously, then, the HIV-infected patient on HAART shows evidence of reconstituting his or her immune system, yet paradoxically, that patient begins to fare worse than he or she did before the HAART was initiated.2 While it is seemingly inexplicable that the patient can worsen despite instituting appropriate HAART, this adverse reaction, known as IRIS, can be explained by the fact the reconstituted immune system is not a reconstituted “normal” immune system—rather it is an exaggerated response.2 As a result, the inflammatory reaction to either subclinical infections or infections that have been previously treated is a pathologic one with an intense cellular proliferative response.2,12 Consequently, extremely immunosuppressed individuals while on their way to immune reconstitution with HAART develop a pathogen-specific immune response that results in excessive tissue inflammation.12 A biphasic immune reconstitution occurs with the first stage characterized by the prompt release of memory T-cells into the circulation and the second stage typified by a gradual rise in naïve T-cell production.1 More specifically within the first 2 weeks of HAART, during the first stage of immune restoration, there is a rapid decrease in the HIV viral load.1 The circulating CD8+ T-cells also rapidly increase.1 Additionally, there is a rise in the number of CD4+ T-cells due to a redistribution of pre-existing memory T-cells caused by a release into the circulation of these cells from lymphoid tissue.1,19 These memory T-cells respond faster to an antigenic stimulus and demonstrate faster effector functions than naïve T-cells, perhaps explaining why a mild OI may result in an exaggerated response.1,19
After 1–1.5 months, there is a proliferation of naïve T-cells from the thymus, which can last up to 2 years and constitutes the second stage of immune restoration, which may be responsible for the continuation of IRIS.1,19 There is also an alteration or imbalance in the proinflammatory T-helper cells (including the Th1 cells, which help clear intracellular pathogens, and the Th17 cells, which help sustain inflammatory responses by producing certain cytokines) and regulatory T-cells, which suppresses effector CD4+ and CD8+ cell proliferation and their cytokine production.8
The homeostatic state cannot be maintained, and a robust inflammatory response develops, which is difficult to contain.8 Consequently, patients may develop recurrence of the initial symptoms associated with their infection or they may develop new inflammatory symptoms following institution of HAART, such as fever, pain from nodal enlargement, and headache.2 Imaging studies in patients with systemic IRIS manifestations may show increasing abnormalities, such as new or worsening lymphadenopathy, enlarging liver, or increasing pulmonary infiltration, all in the face of cultures that are often negative.2 Necrotizing lymphadenitis, disseminated infection from Mycobacterium tuberculosis or Mycobacterium avium complex, may be seen2 and may give insight into CNS-IRIS.
Concerning IRIS terminology, the robust inflammatory reaction to a persistent antigen has been termed “paradoxical” IRIS or, as suggested by Johnson and Nath, “delayed” IRIS.1 In this scenario, the antigen has been previously identified and treated.1,20 However, when the intense inflammatory response is a reaction to a viable pathogen related to a latent infection, the term “unmasking” IRIS or “simultaneous” IRIS has been used.2,5,20 With respect to the incidence of IRIS, in a cohort of 180 HIV-infected individuals on HAART who were coinfected with Mycobacterium tuberculosis, Mycobacterium avium complex, or Cryptococcus neoformans, IRIS occurred in 31.7%, with a 27-day median time between treating the OI and the onset of HAART.2 While in most patients in this particular cohort, the onset of IRIS occurred within 60 days,2 IRIS onset was seen in some patients up to 2 years after the institution of HAART.2 Surprisingly, the long-term outcome of those patients who developed IRIS was generally favorable, with immune reconstitution and viral suppression seen typically after 24 months.2 An increase in CD4 cell count of 100 × 106 cells/L over baseline and an HIV-1 RNA level of <400 copies/mL at 24 months was defined in this study as successful immune restoration.2 Interestingly enough, while aggressive short-term therapy such as corticosteroid administration was needed in some patients to minimize the effects of IRIS, the long-term outcome was typically good.2 Nevertheless, the immune dysfunction that causes IRIS can persist in some individuals.
In another investigation that included a systematic review and a meta-analysis, 1699 patients or 12.97% from 54 cohort studies were reported to have developed IRIS of a total of 13,103 patients started on ART.5 Pooled cumulative incidences were then calculated by specific disease processes in patients with previously confirmed AIDS-defining illnesses.5 Those IRIS incidences were as follows: 37.7%, cytomegalovirus retinitis; 19.5%, cryptococcal meningitis; 16.7%, PML; 15.7%, tuberculosis; 12.2%, herpes zoster; and 6.4%, Kaposi's sarcoma.5 Among unselected patients in whom ART was initiated, IRIS of any type was diagnosed in 16.1%, with 4.5% of those succumbing to this syndrome.5 However, when patients were selected according to disease process, the percentages of those dying changed.5 In patients with cryptococcal meningitis–associated IRIS, 20.8% died in contrast to 3.2% of patients in whom IRIS was associated with tuberculosis.5 From these statistics, it is evident then that the incidence as well as severity of the reaction and the mortality rates of IRIS vary with the specific type of patient population being studied, the type of AIDS-defining illness, and also the geographic locale (global location) in which these patients reside. These differences as well as the paucity of investigations dealing with large patient populations with IRIS and the evolving definitions of IRIS make it difficult to globally standardize and optimize the diagnosis and treatment of patients with IRIS. Nevertheless, investigators are searching for biomarkers for CNS-IRIS such as elevated plasma interleukin 6 levels, certain cytokine profiles, and genetic markers with profiles of gene expression for diagnosing and monitoring IRIS.1
While IRIS can affect any organ in the body, such as the lungs, liver, and lymph nodes, it uncommonly targets the CNS,7 where it has an incidence ranging from only 0.9 to 1.5%.1,21 Nevertheless, when CNS-IRIS develops, it can have a serious impact on patient morbidity and mortality. Mortality rates can range from 5% up to 15%.15,21,22 At autopsy or at brain biopsy, the typical pathology in CNS-IRIS has been characterized by a CD8+ T-cell lymphocytosis with CD8+ cells found in a perivascular and even in a parenchymal location,16,23,24 leading to encephalitis. The relative paucity of CD4+ cells in the brain despite a rising peripheral CD4 cell count in patients on HAART has led Gray et al23 to postulate that the underlying etiology responsible for IRIS is a dysregulated CD8+/CD4+ lymphocyte ratio. In the 8 fatal cases reported by Gray et al,23 CD4 cells, while increasing in the periphery, did not cross the blood-brain barrier, explaining the absence of CD4+ cells in the brain in most cases.16
While recognition of CNS-IRIS both from a clinical and imaging standpoint can be quite difficult because of all its diverse presentations and because it results from a pathogen-specific antigenic response, it is essential to recognize this syndrome so that appropriate therapy can be initiated. Therefore, this review will mainly focus on HIV-associated CNS-IRIS and how to recognize the various expressions of CNS-IRIS, focusing especially on imaging characteristics in PML-IRIS and in cryptococcal meningitis–IRIS.
Common Pathogens Associated with CNS-IRIS
Virus:
PML-IRIS in HIV+ Patients on HAART. It has been reported that in 18% of HIV+ patients with PML, an opportunistic infection caused by the human JC virus, a polyoma virus, PML-IRIS may develop in those treated with HAART.15,47 Depending on the method of diagnosis, however, this figure may be 50% or higher (personal communication, D. Clifford, January 24, 2012). These figures are notable because while some PML-IRIS cases are mild and resolve with continued HAART, other cases may lead to significant morbidity and even mortality because of a severe inflammatory response characterized histopathologically by a marked influx of CD8+ T-cell lymphocytes and macrophages in the areas of demyelination and inflammatory reaction.6,15,37,48⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–61 In fact, in 2 cases, PML-IRIS proved fatal after only 2 weeks of HAART.6 Presumably, the vast outnumbering of CD8+ T-cell lymphocytes compared with CD4+ T-cell lymphocytes may produce an uncontrolled inflammatory response that could prove fatal.6 Because JCV-specific CD4+ T-cell lymphocytes are also known to play a role in the containment of PML, their paucity and the markedly altered CD8+/CD4+ ratio are also contributory factors.6 The use of early and prolonged steroids has been suggested as a means of combating this exaggerated inflammatory response to either the detectable or latent JC virus infection.15
In contrast to PML-IRIS, PML untreated by HAART, when caused by reactivation of the latent and ubiquitous JC virus due to the synergistic effect of HIV,47,63,64 typically results in demyelination, necrosis, and cell death because of a noninflammatory lytic reaction arising from the infection by the virus of the oligodendrocytes and astrocytes.15 It is the T-cell immune deficit caused by HIV that is hypothesized to allow rearrangement of the regulatory region in JCV DNA, which leads to the virus becoming neurotropic and gaining entry to the brain.15 The virus, which is released from the bone marrow or lymphoid tissue stores, is thought to travel hematogeneously to the brain, most probably in B-cells or their precursors.15 Unfortunately, to date, no effective therapy that directly targets the JC virus has been developed.
With the institution of HAART, however, the prognosis for HIV-associated PML has been shown to improve, as in a study of 25 such patients in whom the median survival time was >46 weeks compared with a 10.6-week median survival time in an AIDS Clinical Trials Group study of PML in HIV+ patients before the advent of HAART.65 Increased survival times also correlated with reductions in HIV RNA viral loads.65 Gasnault et al66 also found, in a study of 81 patients with AIDS and PML, a significant survival benefit in those treated with combined antiretroviral therapy. In a different investigation, a median duration of 2.2 years on HAART was found in 63.6% of HIV+ patients with PML, with half of those showing neurologic improvement.64,67 This increased patient survival has been directly linked, in a report by Katz-Brull et al,68 to the degree of inflammatory response mounted by the patient. Katz-Brull et al68 postulated that because disease progression in PML could be mitigated by the inflammatory reaction induced by CD8+ cytotoxic T lymphocytes specific for the JC virus, they could use myo-inositol, a glial marker, as measured by proton MR spectroscopy, as a surrogate marker for brain inflammation and, therefore, as a prognostic tool. Those patients with PML with higher ratios of myo-inositol-to-creatine levels as well as the presence of JC virus–specific cytotoxic T lymphocytes in the blood appeared to have their PML progression limited by this inflammatory reaction, resulting in increased patient survival.68 The cytokines produced by the CD8+ T-cell lymphocytes were postulated as causing this elevation in myo-inositol by inducing an increase in glial cell size and content, leading to a more robust inflammatory response.68
Other authors have found proton MR spectroscopy useful as well.69,70 For example, a study by Chang et al70 of HIV+ patients with PML on HAART found that those patients with higher myo-inositol levels on proton MR spectroscopy had higher survival rates. Yet another positive predictor value for increased survival in those with PML on HAART was found by Berger et al58 to be the presence of lesional contrast enhancement on MR imaging. These imaging findings predictive of improved patient outcome were also found in an MR imaging study by Thurnher et al71 of the initial and follow-up MR imaging findings in AIDS-related PML treated with HAART. This investigation demonstrated that a transient increase in high FLAIR signal and contrast enhancement in the white matter and subsequent MR imaging findings of leukomalacia and atrophy correlated with increased survival (Fig 1).71 Yet another measurable benefit for patients with PML on HAART was found by Usiskin et al,72 who demonstrated white matter anisotropy restoration with treatment.72,73 Unfortunately, however, despite the fact that 10%–50% of patients with PML have their 1-year survival rate increased by HAART, 50% of those patients still die.74 Furthermore, patients with AIDS being treated with HAART may subsequently develop PML.6
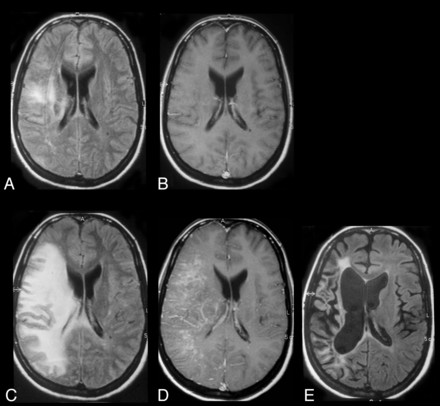
PML-IRIS. Patient with AIDS and PML whose initial MR imaging on axial FLAIR (A) and contrast T1WI (B) shows subcortical and deep white matter lesions due to PML, evidenced by high FLAIR signal without any enhancement. One month later, after HAART initiation, a marked increase in FLAIR high signal (C) compatible with interstitial edema, mass effect, and on contrast T1WI parenchymal and perivascular enhancement (D) develops compatible with PML-IRIS. Long-term follow-up MR imaging with axial FLAIR (E) shows resolution of most of the high-signal abnormalities and atrophy with cortical sulcal and ventricular dilation and no enhancement (not shown). Figures were reproduced with permission from Thurnher et al.71
As for the considerable number of patients on HAART who develop PML-IRIS, the robust inflammatory response that typifies PML-IRIS may be seen any time between 1 week and 26 months after HAART initiation, but most commonly at 3 months,15,73 perhaps due to the restoration of T-cell function peaking at this time.6 This wide time range in which PML-IRIS may develop has been postulated to be related to the initial redistribution in the first several weeks of pre-existing memory T-cells followed 1 month to 4 years later by the proliferation of naïve T-cells.73 In trying to determine what factors can be used to indicate a better prognosis in those patients with known PML-IRIS, a recent investigation of the cellular immune response to the JC virus measuring both CD4+ and CD8+ T-cells via proliferation assays to the JCV antigen and via JCV peptide stimulation showed that the JC-specific CD8+ T-cell response was significantly lower in the PML-IRIS progressors versus the PML-IRIS survivors as was the detectable CD4+ T-cell response.75 Because it is the JC virus–specific cytotoxic CD8+ T lymphocytes that induce an avid cellular immune response, it is these lymphocytes that help contain PML.6
In another study, one consisting of 54 patients with PML-IRIS, the patients who fared worse, having shortened survival rates (2.5 weeks versus 8.5 weeks) and increased mortality, were those whose pre-existing PML worsened after HAART initiation, who developed IRIS earlier on, and who had higher MR imaging lesion loads, compared with those patients who developed IRIS simultaneously with PML.15 Increased survival in this same report was associated with earlier and more prolonged use of steroids as well as contrast enhancement on imaging studies15; 87.5% of those patients with a good outcome demonstrated lesional contrast enhancement on either CT or MR imaging versus 80% with poor outcome whose imaging demonstrated no contrast enhancement.15 In 2 other studies, this perilesional contrast enhancement and its intensity were found to be correlated with the sites and severity of brain inflammation at brain biopsy.15,76,77 It appears then that some degree of inflammatory response following HAART is a good thing, whether associated with the IRIS phenomenon or not. However, if the inflammatory response becomes excessive and uncontrollable, morbidity and mortality rates increase, unless mitigated by medical therapy such as steroids, because it is likely that much of the current mortality of PML is linked to IRIS rather than to progressive JC virus–driven disease.
What now makes PML-IRIS more recognizable than IRIS associated with some other opportunistic diseases, in addition to atypical clinical findings, is the presence of neuroimaging abnormalities that are not classic for untreated PML. While untreated PML typically presents as white matter lesions, often subcortical, low on T1WI, and high on FLAIR and T2WI (due to the myelin destruction), without mass effect and without contrast enhancement with no diffusion restriction centrally (but only peripherally at the active site of lesion expansion with cytotoxic edema),62,78 PML-IRIS is characterized by the development of contrast enhancement of the PML lesions as well as mass effect and increased high FLAIR/T2 signal due to interstitial edema (Figs 2⇓–4).15,58,71,73,76⇓⇓⇓–80 Usually occurring within 1–2 months of HAART1,30 (though they can occur up to 2 years), patchy white matter lesions with multiple areas of nodular enhancement on MR imaging can be seen, which can respond to steroids.1 The white matter lesions can be confined to the posterior fossa, as was the case in 3 of 8 patients with PML-IRIS reported in the literature.30 The peripheral enhancement of the white matter lesions and perivascular spaces has been related to their infiltration by CD8+ T-cells, sometimes accompanied by macrophages and CD4+ T-cells.1,30
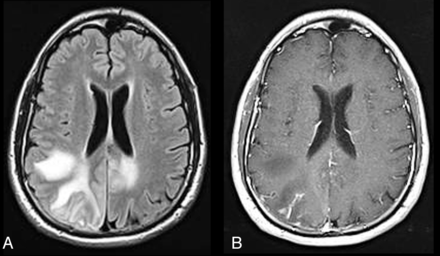
PML-IRIS. HIV-infected patient on HAART with axial FLAIR (A) showing multiple hyperintense asymmetric lesions in the white matter bilaterally and axial postcontrast T1WI (B) showing some patchy enhancement in the right parietal region due to PML-IRIS. Significant response to corticosteroid therapy confirms IRIS.
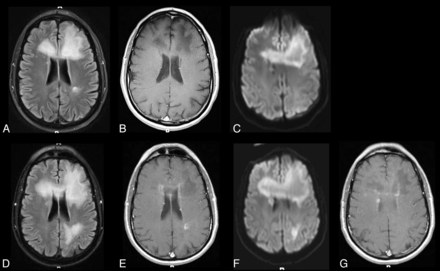
PML-IRIS. HIV-infected patient with personality changes and dysphasia on antiretroviral therapy but noncompliant. Axial FLAIR (A) shows predominantly bifrontal hyperintense white matter lesions with matching low signal on axial postcontrast T1WI (B) without enhancement and with some peripheral restricted diffusion on axial DWI images (C), consistent with PML. Five weeks later following initiation of maraviroc, MR imaging demonstrates progression of the white matter lesions on axial FLAIR (D), the development of some mild patchy enhancement at multiple sites evident on axial gadolinium MR imaging (E and G), and increasing and new areas of peripheral restricted diffusion on axial DWI (F) compatible with IRIS. The patient was placed on steroid therapy to decrease the inflammatory response.
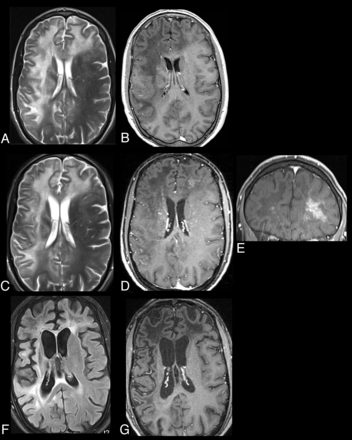
PML-IRIS. AIDS patient with hemiparesis, aphasia, and disorientation. CSF polymerase chain reaction positive for the JC virus (CD4 count, 15 cells/μL). Initial MR imaging pre-HAART with axial T2WI (A) and contrast T1WI (B) showing typical PML lesions with asymmetric white matter hyperintensities in the subcortical and deep white matter in the frontal, parietal, and temporal lobes without mass effect and without enhancement and with matching low signal intensities on the T1WI. Two weeks later after HAART initiation, in addition to the white matter hyperintensities seen on axial FLAIR (C), on contrast MR T1WI with axial (D), and coronal (E) views, perivascular and parenchymal enhancement is now seen bilaterally, greatest in the left frontal lobe. Nine months later, axial FLAIR (F) and contrast T1WI (G) demonstrate resolution of the enhancement, decrease in the white matter hyperintensities, and development of atrophy.
If this intense inflammatory response with edema, contrast enhancement, and mass effect can subsequently be mitigated, patients may have an improved outcome. However, not all patients with HIV-associated PML-IRIS demonstrate contrast enhancement of the PML lesions.30 Contrast enhancement may be seen in only 56% of patients.73 Indeed, much of the underestimation of PML-IRIS results from the assumption of clinicians that it only occurs when contrast enhancement is seen in PML lesions. In fact, this enhancement may be a late and extreme consequence, with substantial and abnormal inflammatory changes in PML lesions occuring well before gadolinium contrast enhancement. Fortunately, a response can be seen with steroids.1,15,81
Yet another tool used to predict patient outcome in HIV-associated PML-IRIS has been diffusion-weighted imaging. A study by Buckle and Castillo74 found that in the clinically rapidly progressive patients with PML-IRIS, the ADC values both centrally and totally as well as the JCV titers pre-HAART were the highest, whereas those with lower ADC values were associated with stable lesions or remyelination. Also while the ADC values in the center of the white matter lesion increased only slightly during a 1-month time period on HAART, in those patients whose PML progressed slowly, there was a significant increase in ADC values in the total lesion and central core in those patients with rapid PML progression who had been on HAART for 1 month.74 The implication is that because PML is a destructive white matter lesion, increased destruction manifested by increased diffusibility on diffusion-weighted imaging would indicate disease progression and hence poorer patient outcome.74
That PML-IRIS can be fulminating and lead to patient death was evident from the case report of Vendrely et al6 of a patient with AIDS with PML started on HAART who subsequently deteriorated neurologically. The patient's MR imaging showed an increase in the number and size of the lesions, all of which enhanced compared with the pre-HAART MR imaging.6 Biopsy showed both demyelinating lesions as well as severe inflammation with massive T-cell lymphocyte and macrophage infiltration without JCV detection.6 Unfortunately, high-dose steroids did not prevent the patient's death. At autopsy, an acute perivenous leukoencephalitis was found, mostly comprised of CD8+ lymphocytes without detectable JC virus in those specific areas. However, areas of abundant JC virus with active PML inflammatory lesions and perivascular and parenchymal infiltration by T lymphocytes were also found.6 While CD8+ lymphocytes were in abundance, CD4+ lymphocytes were absent. The patient's death with PML-IRIS was thought then to be related to a dysregulation of the immune response with an imbalance in the CD8+/CD4+ T-cell ratio.6 The marked infiltration of CD8+ T-cell lymphocytes into the brain parenchyma was not matched by a sufficient enough CD4+ T-cell lymphocyte response.6 This led to a perivenous leukoencephalitis as well as an aggravation of the JCV infection.6
Virus:
PML-IRIS in HIV-Negative Patients on Immunomodulatory Therapies. While not the focus of this article, a brief mention should be made of the fact that in HIV-negative patients such as those with autoimmune diseases treated with immunomodulatory therapies, in organ transplant patients, or in those with hematologic malignancies, PML can occur.47 For example, in patients with multiple sclerosis or Crohn disease treated with immunomodulatory medications such as natalizumab (an α4 β1 and α4 β7 integrin inhibitor that binds α-integrin molecules on the surface of T- and B-cells), PML can develop, albeit rarely.47,64,73 Since the integrins serve as attachment ligands for the vascular cell adhesion molecules on endothelial cells, natalizumab, by preventing the binding of the integrin onto the vascular cell adhesion molecule, causes a loss in immune surveillance because the T-cells can no longer gain access to the brain.47 This complication of PML occurring with biologically immune-modifying therapies82⇓⇓⇓⇓–87 has been found to occur during the first 3 years of exposure to natalizumab.83 In the first 2 years on this therapy, the incidence has been cited at 1 in 1133.64 If subsequently that treatment is terminated and plasmapheresis is performed, the increased trafficking of leukocytes into the CNS can result in PML-IRIS.64,82 With plasma exchange, which increases the clearance of natalizumab, clinical symptoms can worsen due to the development of PML-IRIS.83
Typical for the IRIS phenomenon, as the patent deteriorates clinically, the PML lesions on MR imaging enlarge and increased gadolinium enhancement can be seen within days to weeks of the plasma exchange.83 The PML-IRIS developing in this particular setting is said to be more severe than that observed in the HIV+ patient with PML-IRIS because of the restored immune surveillance.83 Neurologic deterioration and even brain herniation and death can occur.83 Steroids have been used to dampen this effect of PML-IRIS. Certain other monoclonal antibody therapies that perturb the immune system, such as rituximab (which targets the CD20 cell-surface marker), used in the treatment of lymphoproliferative disease (typically B-cell malignancies), rheumatoid arthritis, and systemic lupus erythematosus, and efalizumab, used for the treatment of psoriasis (which binds CD11), have also been shown to have an increased risk for PML development and, following cessation, increased risk for PML-IRIS.64
Fungus: Cryptococcal Meningitis–IRIS
Cryptococcus neoformans is an organism that can cause infection frequently seen in association with IRIS.88 Cryptococcal-IRIS can be manifested in many different ways—as lymphadenitis, pneumonitis, cryptococcal meningitis, or cryptococcomas—and can result in considerable morbidity and mortality.20,89 In CM-IRIS, mortality rates have ranged between 8% and 30%.20 In fact, according to some investigators, the morbidity and mortality rates have actually increased in CM-IRIS.20 For example, in a prospective study of 65 HIV-positive patients with proved cryptococcal meningitis on antifungal medication (amphotericin B) who were ART-naïve, IRIS-associated cryptococcal meningitis developed in 17% (11 patients) at a median of 29 days after the initiation of ART.20 While there was a greater immune restoration (as measured by a greater CD4 rise from baseline after 6 months) noted in patients with CM-IRIS as opposed to those with CM without IRIS, there was also a higher mortality rate in the patients with CM-IRIS (4/11 versus 14/54).20 There was a trend for those patients developing CM-IRIS to have a higher fungal burden at the end of ≥7 days of initial treatment with amphotericin B.20 In another investigation, a prospective study of 101 Ugandans with AIDS without any prior ART exposure who then developed cryptococcal meningitis after ART initiation, IRIS developed in a median time of 8.8 weeks in 45%, with 30% exhibiting CNS symptoms.90 Thirty-six percent of those with CM-IRIS died, compared with 21% with CM without IRIS.90
In a search for serum biomarkers in CM-IRIS that might lead to more advantageous treatment regimens, it was found that the pre-ART serum cryptococcal antigen level was 4 times higher in those developing CM-IRIS.90 Furthermore, a paucity of proinflammatory cytokine responses pre-ART, evidenced by lower tumor necrosis factor α, lower vascular endothelial growth factor, lower granulocyte-macrophage colony–stimulating factor, and lower granulocyte colony–stimulating factor combined with heightened Th17 and Th2 responses as measured by higher levels of interleukin 4 and interleukin 17, was predictive of future IRIS.90 The authors postulated that these biomarkers could be used to determine when to initiate ART or to guide other interventional therapies.90 With patients on ART these authors also found increasing levels of D-dimer and C-reactive protein to be biomarkers pointing to an inflammatory response.90
Patients with CM-IRIS can be recognized clinically by the development of headache, fever, malaise, altered mental status, raised intracranial pressure, and cranial nerve palsies in the setting of lymphadenopathy and new pulmonary infiltrates.91 Cavitary lung lesions, suppurative mediastinal lymph nodes, and meningismus due to the exaggerated local inflammatory responses from increased reactivity to the cryptococcal antigen and higher cryptococcal antigen titers, a higher fungal burden in the blood, higher opening pressures in the CSF, and sometimes culture-negative CSF are diagnostic clues that may differentiate CM-IRIS from pre-HAART cryptococcal infection.20,25,92 Initiating antiretroviral therapy within 1–2 months of the diagnosis of CM20 and CD4 counts below 11 cells/mm3 as well as higher baseline HIV RNA levels have also been viewed as risk factors for CM-IRIS.25
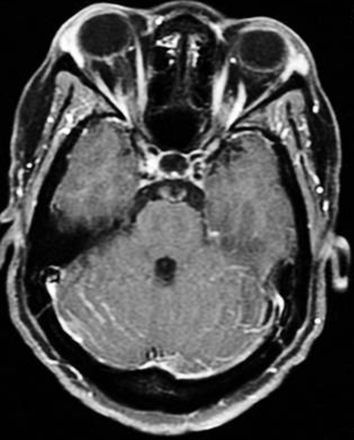
Concerning neuroimaging, certain striking differences have been found in those with CM-IRIS as opposed to HAART-naïve HIV-infected patients with CM. Before the advent of HAART, leptomeningeal enhancement in CM was uncommon in patients with AIDS because those individuals were unable to mount a sufficient inflammatory response.93 However, with HAART and CM-IRIS, an intense inflammatory reaction can be seen. Because of a restoring immune system, CT or MR imaging can demonstrate leptomeningeal enhancement (Fig 5), which can be accompanied by a communicating hydrocephalus in CM-IRIS. The findings of linear perivascular enhancement in the sulci and new meningeal or choroid plexus enhancement have been shown to be imaging indicators of CM-IRIS.19,90,94 In a case illustrated by Riedel et al,19 a cerebellar lesion with high FLAIR signal having mass effect on the fourth ventricle was seen to develop in association with an increase in meningeal enhancement 2 weeks after an HIV-infected patient with CM was treated with antiretroviral therapy.
Cryptococcal meningitis–IRIS. Axial T1-weighted image with contrast shows enhancement along the folia of the cerebellum and the meninges in a patient with HIV infection and cryptococcal meningitis who was treated with amphotericin B and then HAART.
While distention of the Virchow-Robin spaces manifested as high T2/FLAIR signal, particularly in the basal ganglia, and gelatinous pseudocysts have been imaging features of cryptococcal meningitis in both the pre- and post-HAART era due to the production of a viscous mucoid material by the acidic polysaccharide capsule of the cryptococcal organism,95 enhancement of these Virchow-Robin spaces appears characteristic of CM-IRIS as does secondary involvement of the brain parenchyma characterized by areas of high T2/FLAIR signal (Fig 6A), restricted diffusion, and parenchymal enhancement. In a report of 2 HIV+ patients with cryptococcal meningitis started on HAART with negative findings on pretreatment MR images, in 1 patient 7 months later, leptomeningeal enhancement was observed and multiple enhancing parenchymal lesions in the cortex compatible with cryptococcomas; whereas in the other patient, focal cortical and subcortical lesions were seen 6 months later.96 In a prospective study by Bicanic et al,20 contrast CT scans were obtained in 4 of the 65 patients with CM-IRIS. Two of these CT scans showed infarcts, either in the basal ganglia bilaterally or in the unilateral basal ganglia and temporal and parietal lobes. While infarcts can certainly cause restricted diffusion, the gelatinous mucoid material produced by the cryptococcal capsule can also restrict diffusion in the parenchyma.97 When there is a sufficient enough inflammatory response induced by IRIS, contrast enhancement can be seen (Fig 6B).
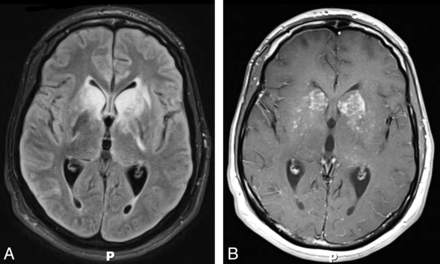
Late cryptococcal meningitis–IRIS. A 29-year-old man with HIV/AIDS and cryptococcal meningitis who was treated successfully. Four months later while on fluconazole and with a CD4 count of 66 cells per microliter and an HIV viral load of 400,000 copies/mL, he was started on antiretroviral therapy (emtricitabine/tenofovir/efavirenz [Atripla]). Eight months after initiation of the antiretroviral therapy, the patient developed headache, stiff neck, nausea, and vomiting. Axial FLAIR (A) and axial T1WI postcontrast (B) imaging show distention of the Virchow-Robin spaces in the basal ganglia with hyperintense signal and enhancement. These images also demonstrate that the inflammatory process has spread into the parenchyma of the basal ganglia, where high FLAIR signal and patchy enhancement are seen. Fungal cultures at this time were negative, and the cryptococcal antigen level was weakly positive. A diagnosis of late IRIS was made, and the patient was started on steroids, to which he responded.
Higher organism burden in the CSF at disease onset in CM-IRIS has also been reported to be associated with elevated intracranial pressure.91,92 This elevated intracranial pressure is due to the blockage of CSF pathways and arachnoid villi by the production of greater amounts of mucoid material, by the higher number of organisms, and by the greater reactivity to the cryptococcal antigens.91 A lumbar drain or ventriculostomy is often necessary to combat the increased mortality (25%) with elevated intracranial pressure that has been reported.91 Because a high opening pressure in patients with CM-IRIS is considered a risk factor for increased mortality, the suggestion has been made to delay for 1 month the institution of HAART in these patients who develop cryptococcal meningitis.91 Treatment with amphotericin B and flucytosine for 2 weeks and fluconazole for 8 weeks has been suggested in this setting.91
One word of caution should be expressed. While the imaging findings mentioned above can be clues to the diagnosis of CM-IRIS, a negative CT or MR imaging finding or one showing only cortical atrophy does not exclude that diagnosis. Several studies have shown significant percentages of patients in whom the MR imaging or CT findings were negative in cryptococcal meningitis.98 In fact, a recent article has shown that DTI may be a useful tool in CM because it can detect changes that may be more widespread than anticipated from conventional MR imaging.99 Investigating the neuropsychological sequelae in HIV-negative patients with cryptococcal meningitis and its correlation to microstructural changes in the white matter, these authors found that higher CSF cryptococcal antigen levels were associated with poorer DTI parameters, with increasing ADC values and decreasing fractional anisotropy values and worse cognitive performance.99 Their conclusion was that a higher fungal burden correlated with a greater microstructural change in the white matter, which was apparent only by DTI and not by routine MR imaging.99 While this study was performed in the HIV-negative population, one can certainly postulate that advanced imaging techniques such as DTI should be very useful in assessing parenchymal damage in CM-IRIS because of the overzealous inflammatory reaction present in some of these patients.
Summary
While CNS-IRIS is a diagnosis of exclusion, the neuroradiologist can be pivotal in the early recognition of this condition because of often atypical MR imaging and CT findings, which characterize this syndrome, as typified in cryptococcal infection and PML. Contrast enhancement, transient increase in parenchymal abnormalities with high signal on FLAIR, mass effect, and restricted diffusion can be the diagnostic imaging clues to CNS-IRIS. In an HIV+ patient whose severe immunosuppression responds rapidly to HAART while neurologic symptoms worsen, neuroimaging can give credence to the diagnosis of CNS-IRIS, thereby aiding the clinician in the medical management of the patient. Ultimately, if the inflammatory response can be contained as the patient's immune system recovers, the patient's long-term outcome can be improved.
Footnotes
Disclosures: Majda Thurnher—UNRELATED: Royalties: Amirsys. David Clifford—UNRELATED: Consultancy: All <$10,000 annually: Biogen Idec, Genentech, Millennium, Genzyme, Bristol Myers Squibb, Pfizer, Janssen, Expert Testimony: Biogen Idec, Comments: European Medicines Agency (EMA) discussion of natalizumab, Payment for Development of Educational Presentations: Millennium, payment for teaching video on exam for PML; Genentech, payment for teaching video on PML diagnosis, Other: Millennium, Independent Adjudication Committee, Genzyme, Data Monitoring Committee, Chair; Genentech, Panel of Experts, Translational Immunology Consultant; Pfizer, Data Safety Monitoring Committee (DSMB).
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- © 2013 by American Journal of Neuroradiology