Abstract
BACKGROUND AND PURPOSE: Cognitive function can improve or decline after carotid endarterectomy. Proton MR spectroscopy can be used evaluate cerebral metabolites, such as N-acetylaspartate, choline, and creatine, in vivo. The purpose of the present study was to determine whether postoperative changes in cerebral metabolites measured by using 3T proton MR spectroscopy were associated with changes in cognitive function after CEA.
MATERIALS AND METHODS: In 100 patients undergoing CEA for ipsilateral cervical internal carotid artery stenosis (≥70%), brain proton MR spectroscopy was performed before and after surgery. NAA/Cr and Cho/Cr ratios were measured in regions of interest placed in the centrum semiovale of both cerebral hemispheres. Neuropsychological testing was also performed preoperatively and 1 month postoperatively. Multivariate statistical analysis of factors related to postoperatively changed cognition was performed, and odds ratios with 95% confidence intervals were calculated.
RESULTS: On the basis of the neuropsychological assessments, 10 (10%), 80 (80%), and 10 (10%) patients were defined as having postoperatively improved, unchanged, and impaired cognition, respectively. A positive and high ΔNAA/Cr ratio (postoperative value–preoperative value) in the cerebral hemisphere ipsilateral to the operative site was significantly associated with postoperatively improved cognition (95% CI, 13.3–21.3; P = .0016). Negative and high absolute values of the ΔNAA/Cr ratio (95% CI, 0.018–0.101; P = .0039) and ΔCho/Cr ratio (95% CI, 0.042–0.135; P = .0046) in the ipsilateral cerebral hemisphere were significantly associated with postoperatively impaired cognition.
CONCLUSIONS: Postoperative changes in cerebral metabolites measured by using proton MR spectroscopy were associated with changes in cognitive function after CEA.
ABBREVIATIONS:
- CEA
- carotid endarterectomy
- CI
- confidence interval
- Rey test
- Rey-Osterreith Complex Figure Test
- WAIS-R
- Wechsler Adult Intelligence Scale Revised
Carotid endarterectomy can reduce the risk of stroke in appropriately selected patients.1,2 While CEA may also improve cognitive function,3,4 cognitive impairment occurs in 10%–30% of patients following CEA.5⇓⇓–8 A recent study reported that 11% of patients undergoing CEA experienced improvement in cognitive function after surgery, while another 11% experienced a decline in cognitive function after surgery.9 Cerebral metabolism may also change along with these postoperative changes in cognitive function, but the relationship between these 2 factors remains unclear.
Proton MR spectroscopy enables noninvasive chemical analysis in vivo, because the proton is the most sensitive stable nucleus for MR spectroscopy and nearly all metabolites contain protons.10 Proton MR spectroscopy can also measure relative changes in metabolites, including N-acetylaspartate, choline-containing compounds, and total creatine.11 Investigators have suggested that the level of NAA in the brain is an index of neuronal viability12 and that the level of choline in the brain is associated with membrane synthesis or degeneration in neural tissues.13 On the basis of these hypotheses, proton MR spectroscopy has been applied for the study of various pathologic conditions of the central nervous system.11,12,14⇓⇓⇓⇓⇓⇓⇓–22 Several studies have also investigated relative changes in NAA and/or choline following CEA by using proton MR spectroscopy.23⇓⇓–26 However, the clinical significance of such postoperative changes remains unknown.
Correlations between MR spectroscopic measures and neuropsychological function have been reported in patients with Alzheimer disease, mild cognitive impairment, idiopathic normal pressure hydrocephalus, and cerebral infarction.14,17⇓⇓⇓⇓–22 Thus, the purpose of the present prospective study was to determine whether a postoperative increase or decrease in cerebral metabolites measured by using proton MR spectroscopy was associated with improvement or impairment in cognitive function after CEA.
Materials and Methods
Inclusion and Exclusion Criteria of Patients
We prospectively selected patients scheduled to undergo CEA and satisfying the following inclusion criteria: 75 years of age or younger; having ipsilateral cervical internal carotid artery stenosis (≥70%) on angiography with MR imaging, CT, or arterial catheterization according to the method of the North American Symptomatic Carotid Endarterectomy Trial1; having preoperative useful residual function (modified Rankin Scale, 0 or 1); the presence of episodes of ipsilateral carotid territory ischemic symptoms that had occurred between 2 weeks and 6 months before presentation to our department (defined as symptomatic carotid stenosis) or the absence of episodes of ipsilateral carotid territory ischemic symptoms or the presence of episodes of ipsilateral carotid territory ischemic symptoms that had occurred >6 months before presentation to our department (defined as asymptomatic carotid stenosis)2; having no infarction in the cerebral cortical area perfused by ≥1 branch of the middle cerebral artery confirmed by MR imaging, including T2-weighted and fluid-attenuated inversion recovery sequences, that was performed 2 weeks before surgery; and obtaining written informed consent. Patients who satisfied the following criteria after surgery were excluded from the present study: new neurologic deficits lasting for 2 weeks after surgery; and additional ischemic lesions on MR imaging, including T2-weighted and FLAIR sequences, performed 2 weeks after surgery compared with preoperative MR imaging. A 1.5T whole-body imaging system (Signa MR/i; GE Healthcare, Milwaukee, Wisconsin) was used for evaluation of ischemic lesions before and after surgery.
All study protocols were reviewed and approved by the local institutional ethics committee.
MR Spectroscopy
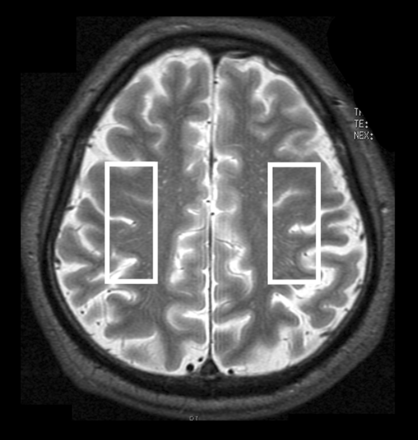
A 3T scanner (Signa Excite HD; GE Healthcare) with a “birdcage” quadrature head coil was used for this study. First, all subjects underwent axial T2-weighted imaging. In the T2-weighted images for each subject, 1 section through the upper gray matter above the centrum semiovale was selected, and a single-voxel region of interest was manually and symmetrically placed in the bilateral cerebral hemispheres so that the proportion of the cortical gray matter and CSF occupying the region of interest was as high and as low as possible, respectively (Fig 1). Voxel size was 17 × 50 × 15 mm3. Next, acquisition of proton MR spectroscopy was performed by using point-resolved spectroscopy with the following parameters: TR, 2000 ms; TE, 144 ms; data size, 4 K points; spectral width, 5000 Hz; 96 acquisitions (3.9 minutes). An area under each peak of 3 main metabolites was automatically obtained on the MR imaging console: choline-containing compounds at 3.2 ppm, total creatine at 3.0 ppm, and NAA at 2.0 ppm. Area ratios for NAA and Cho peaks were expressed as the relative ratio to Cr in each spectrum.
Regions of interest placed on 1 section through the upper gray matter above the centrum semiovale in an axial T2-weighted MR image to obtain MR spectroscopy.
Patients underwent MR spectroscopy within 7 days before surgery and between 2 and 4 weeks after surgery. On each occasion, care was taken to place the region of interest in the same position on the T2-weighted image. NAA/Cr and Cho/Cr ratios in each cerebral hemisphere were calculated before and after surgery, and the difference between these 2 values (postoperative values minus preoperative values) was calculated and defined as ΔNAA/Cr and ΔCho/Cr ratios, respectively.
Neuropsychological Evaluation
For each patient, a battery of neuropsychological tests was administered, consisting of the Japanese translation of the Wechsler Adult Intelligence Scale Revised,27 the Japanese translation of the Wechsler Memory Scale,28 and the Rey test.29 WAIS-R generates a verbal and performance intelligence quotient. The Rey test evaluates copy and recall of a complex figure. Thus, 5 scores (WAIS-R verbal IQ, WAIS-R performance IQ, Wechsler Memory Scale, Rey copy, and Rey recall) were used to evaluate cognitive function.
The neuropsychological tests were performed within 7 days before surgery and were repeated 1 month after surgery. All examinations were administered by a trained neuropsychologist who was blinded to the patient clinical information.
Postoperative cognition was categorized as improved, unchanged, or impaired for each patient on the basis of the definition described previously.9 Briefly, 40 healthy volunteers served as controls and underwent the same neuropsychological tests on 2 separate occasions (intertest interval, 1–2 months).9 Differences in each neuropsychological test score between the 2 tests (the second test score–the first test score) were calculated. For the neuropsychological test scores of patients undergoing CEA, a significant increment was defined as a postoperative test score greater than the preoperative score plus the value of the mean minus 2SDs of the difference between the 2 test scores in the controls; a significant decrement was defined as a postoperative test score less than the preoperative score minus the absolute value of the mean: 2SDs of the difference between the 2 test scores in the controls. A patient was defined as having postoperative cognitive improvement or impairment when there was a significant increment or decrement in ≥1 postoperative neuropsychological score, respectively; a patient was defined as having an unchanged cognition after surgery when there was no significant increment or decrement in any postoperative neuropsychological scores.9
Intraoperative Management
All patients underwent surgery under general anesthesia. An intraluminal shunt during ICA clamping was not used in any of the patients. The mean duration of ICA clamping was 37 minutes (range, 26–49 minutes).
Statistical Analysis
Data are expressed as the mean ± SD. Changes between the pre- and postoperative NAA/Cr or Cho/Cr ratio were evaluated by using the Wilcoxon signed rank test. Differences in the change of each neuropsychological test score among the controls and patients were evaluated by using the Mann-Whitney U test. Differences or incidences of each baseline characteristic among the 3 groups (patients with postoperatively improved, unchanged, or impaired cognition) were evaluated by using the χ2 test followed by the Bonferroni inequality correction or the Scheffe F test. A multivariate statistical analysis of factors related to postoperatively improved or impaired cognition relative to postoperatively unchanged cognition was also performed by using a logistic regression model with odds ratios with 95% CIs calculated. Variables with P < .2 in the univariate analyses were selected for analysis in the final model. For all statistical analyses, significance was set at the P < .05 level. The only exception was the χ2 test followed by the Bonferroni inequality correction, in which differences were deemed statistically significance if P < .05/3 = 0.0167.
Results
During 36 months, 136 patients underwent CEA. Of these, 104 patients preoperatively satisfied the inclusion criteria. However, 2 patients experienced new major neurologic deficits that lasted 2 weeks after surgery; another 2 patients developed additional asymptomatic ischemic lesions on MR imaging, including T2-weighted and FLAIR sequences performed 1 month after surgery, compared with preoperative MR imaging. These 4 patients did not undergo MR spectroscopy and neuropsychological testing after surgery and were excluded. Thus, the remaining 100 patients were analyzed in the present study. None of these patients experienced further ischemic symptoms between initial evaluation and surgical intervention.
The mean age of the 100 patients (89 men, 11 women) studied was 68 ± 6 years (range, 52–75 years). Concomitant disease states and symptoms were recorded, including 88 patients with hypertension, 37 patients with diabetes mellitus, and 53 patients with dyslipidemia. Sixty-four patients had ipsilateral symptomatic ICA stenosis, and the remaining 36 patients had asymptomatic ICA stenosis. Preoperative MR imaging demonstrated infarction in the hemisphere ipsilateral to the ICA stenosis in 54 patients and no infarction in 46 patients. The overall average degree of ICA stenosis was 87.3 ± 8.8% with a range of 70%–99% according to the method of the North American Symptomatic Carotid Endarterectomy Trial.1 The contralateral ICA was occluded in 6 patients, and 8 additional patients had 60%–99% stenosis.
The mean ± SD of NAA/Cr and Cho/Cr ratios in each cerebral hemisphere before and after CEA among 100 patients is summarized in Table 1. When we analyzed them as a group, none of these values differed between measurements before and after surgery.
NAA/Cr and Cho/Cr ratios before and after surgerya
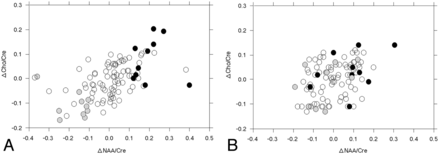
Figure 2 shows the distribution of ΔNAA/Cr and ΔCho/Cr ratios in each patient in each cerebral hemisphere. The ΔNAA/Cr ratio ranged from −0.370 to 0.402 or from −0.190 to 0.303 in the cerebral hemisphere ipsilateral or contralateral to the operative site, respectively. The ΔCho/Cr ratio ranged from −0.170 to 0.204 or from −0.130 to 0.140 in the cerebral hemisphere ipsilateral or contralateral to the operative site, respectively.
Distribution of ΔNAA/Cr and ΔCho/Cr ratios in each patient in the cerebral hemisphere ipsilateral (A) or contralateral (B) to the operative site. Closed, open, and half-tone circles indicate patients with postoperatively improved, unchanged, and impaired cognition, respectively.
On the basis of the neuropsychological assessments performed before and after surgery, 10 (10%), 80 (80%), and 10 (10%) patients were regarded as having postoperatively improved, unchanged, and impaired cognition, respectively. Table 2 shows differences in each neuropsychological test score between the 2 tests (the second test score–the first test score) in controls and patients. While none of these discrepancies differed between the controls and all patients or between controls and patients without a postoperative change in cognition, all the differences were higher in patients with postoperatively improved cognition and lower in those with postoperatively impaired cognition compared with the controls.
Differences in each neuropsychological test score between the 2 tests (the second test score–the first test score) in controls and patientsa
Table 3 shows the univariate analysis of patient characteristics among the 3 groups. The ΔNAA/Cr and ΔCho/Cr ratios in the cerebral hemisphere ipsilateral to the operative site statistically differentiated patients with postoperatively improved, unchanged, and impaired cognition. Specifically, ΔNAA/Cr and ΔCho/Cr ratios were higher in patients with postoperatively improved cognition and lower in those with postoperatively impaired cognition compared with patients without a postoperative change in cognition (Fig 2A). In addition, the ΔNAA/Cr ratio in the contralateral cerebral hemisphere was higher in patients with postoperatively improved cognition than in those with postoperatively unchanged or impaired cognition (Fig 2B). Other variables did not differ when comparing patients with postoperatively improved, unchanged, or impaired cognition. After we eliminated closely related variables in the univariate analyses, the following confounders (P < .2) were adopted in the logistic regression model for the multivariate analysis: age; ΔNAA/Cr ratio in each cerebral hemisphere; ΔCho/Cr ratio in the ipsilateral cerebral hemisphere for postoperatively improved cognition relative to postoperatively unchanged cognition; and ΔNAA/Cr and ΔCho/Cr ratios in the ipsilateral cerebral hemisphere for postoperatively impaired cognition relative to postoperatively unchanged cognition. Subsequent multivariate analysis revealed that positive and high ΔNAA/Cr ratios in the ipsilateral cerebral hemisphere significantly correlated with postoperatively improved cognition (95% CI, 13.3–21.3; P = .0016) and that negative and high absolute values of the ΔNAA/Cr ratio (95% CI, 0.018–0.101; P = .0039) and the ΔCho/Cr ratio (95% CI, 0.042–0.135; P = .0046) in the ipsilateral cerebral hemisphere significantly correlated with postoperatively impaired cognition.
Comparison of characteristics among patients with postoperatively improved, unchanged, or impaired cognition
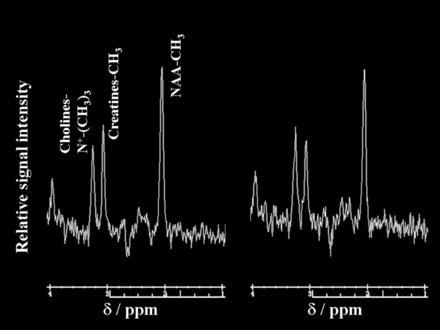
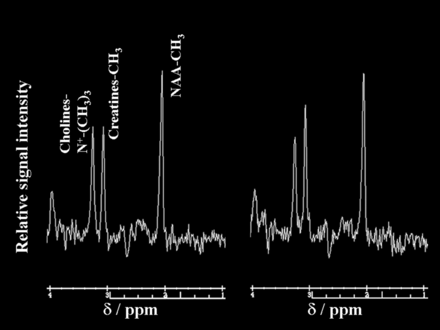
Figures 3 and 4 show proton MR spectra in each patient with postoperative improvement or impairment in cognitive function, respectively.
Proton MR spectra obtained by using point-resolved spectroscopy in the region of interest in the cerebral hemisphere ipsilateral to the operative site in a 62-year-old man with improved cognition after endarterectomy for symptomatic right internal carotid artery stenosis. Area for the NAA or choline peak to the area for the creatine peak is relatively increased after surgery (NAA/creatine, 1.83; choline/creatine, 1.06) (right graph) compared with the preoperative spectrum (NAA/creatine, 1.56; choline/creatine, 0.86) (left graph).
Proton MR spectra obtained by using point-resolved spectroscopy in the region of interest in the cerebral hemisphere ipsilateral to the operative site in a 72-year-old man with impaired cognition after endarterectomy for symptomatic left internal carotid artery stenosis. The area for the NAA or choline peak to the area for the creatine peak is relatively reduced after surgery (NAA/creatine, 1.33; choline/creatine, 0.83) (right graph) compared with the preoperative spectrum (NAA/creatine, 1.57; choline/creatine, 0.99) (left graph).
Discussion
The present study demonstrated that postoperative changes in cerebral metabolites measured by using proton MR spectroscopy were associated with changes in cognitive function following CEA.
Several studies have investigated changes in NAA/Cr and/or Cho/Cr ratios following CEA by using proton MR spectroscopy. While most of these studies reported increases in these values after CEA,23⇓⇓–26 the remainder found no changes.30 The variation in results between these studies may be from differences in methodologic factors, including the number of patients, types of patient, types of MR scanners, and the timing of postoperative assessment. In particular, previous studies have been performed by using devices operating at 1.5T and included a relatively smaller sample size (≤20 subjects).23⇓⇓–26,30 The main advantages of proton MR spectroscopy at 3T over that at 1.5T include a higher signal-to-noise ratio, higher spatial and temporal resolutions, and better spectral resolution.11 These advantages allow acquisitions of higher quality and result in a higher sensitivity for the detection of nervous system metabolites.11 In the present study using a 3T imager and a large sample size, NAA/Cr and Cho/Cr ratios did not differ between measurements before and after CEA when they were analyzed as a group. Thus, NAA/Cr and Cho/Cr ratios usually do not change after CEA.
While numerous studies investigated changes in cognitive functioning following CEA by using neuropsychological testing,3⇓⇓⇓⇓–8 there were no clear guidelines for determining significant improvement or impairment in cognition. This is because such changes may, in part, reflect the “practice effect” (an improvement in scores when patients are repeatedly tested).3,7 In contrast, physicians or patients' families or both often report subjective postoperative improvement or impairment in cognition for patients undergoing CEA.9 A recent study demonstrated that the optimal cutoff point of the degree of postoperative increase or decrease in neuropsychological test scores, such as the WAIS-R verbal IQ, WAIS-R performance IQ, Wechsler Memory Scale, Rey copy, and Rey recall, in detecting subjective improvement or impairment in cognition after surgery is identical to the mean + 2SDs or the mean − 2SDs, respectively, of the control value obtained from healthy subjects.9 Furthermore, of patients with a postoperative increase in test scores more than the upper cutoff point or of those with a postoperative decrease in test scores less than the lower cutoff point in ≥1 neuropsychological test, 90% of patients in each group exhibited subjectively improved or impaired cognition, respectively, after surgery.9 All patients with postoperative increases or decreases in test scores between the upper and lower cutoff points in all neuropsychological tests exhibited subjectively unchanged cognition after surgery.9 Thus, in the present study, we determined significant postoperative improvement and impairment in cognition for each patient by using the same definition. As a result, 10% of patients who underwent CEA were defined as having postoperative improvement in cognition, and another 10% of patients who underwent CEA were defined as having postoperative impairment in cognition. These incidences were consistent with those from previous studies.9
The present study demonstrated that a postoperative increase and decrease in NAA/Cr ratios in the cerebral hemisphere ipsilateral to operative site were significantly associated with postoperative improvement and impairment in cognition, respectively. NAA is an amino acid found almost exclusively in neuronal cells, and the level of NAA in the brain may be an index of neuronal viability.12 The NAA/Cr ratio also correlates with cerebral oxygen metabolism of the gray matter in patients with steno-occlusive carotid artery disease.16 In addition, a decrease in the NAA/Cr ratio is associated with a decrease in cognitive function in elderly populations,18,21 patients with cerebral infarction,17,22 and those with Alzheimer disease.20 Thus, the present data suggested that neuronal damage caused by CEA results in postoperative cognitive impairment. In contrast, in patients with idiopathic normal pressure hydrocephalus, a significant increase in the NAA/Cr ratio following shunting is related to postoperative cognitive improvement,19 which is consistent with observations from the present study. Therefore, a reduction in NAA may be reversible, and the level of NAA can recover with cognitive improvement, probably resulting from restoration of brain perfusion following surgery.
A postoperative decrease in Cho/Cr ratios in the ipsilateral cerebral hemisphere was significantly associated with postoperative impairment in cognition. The level of choline in the brain may be associated with membrane synthesis or degeneration in neural tissue.13 Elevation of the choline signal has been demonstrated in patients with multiple sclerosis,15 in those with severe vasospasm after subarachnoid hemorrhage,31 and in patients with acute ischemic stroke.32 In such pathologic conditions, damage to neural tissue may be ongoing, resulting in an increase in myelin membrane degeneration products and leading to elevation of the choline signal. In contrast, the choline level detected on proton MR spectroscopy is decreased in the ischemic cores and in the surrounding tissue that otherwise appears normal on MR imaging performed from 5 to 30 days after the onset of massive cerebral infarction.33 In this subacute stage of ischemic stroke, damage to the neural tissue is probably complete, and myelin membrane synthesis and degeneration may be reduced, resulting in a decrease in the choline signal. Cho/Cr ratios correlated positively with scores of neuropsychological testing in the elderly population18,21 and in patients with preclinical Huntington disease.34 In the latter, investigators have suggested that a decrease in Cho indicates a reduction of myelin membrane turnover that precedes neuronal death that may be responsible for the neuropsychological deficits.34
Thus, a postoperative decrease in the Cho/Cr ratio as well as a postoperative decrease in the NAA/Cr ratio may imply neuronal damage caused by CEA, thereby resulting in postoperative cognitive impairment. On the other hand, the univariate analyses showed that the ΔCho/Cr ratio in the cerebral hemisphere ipsilateral to the operative site was significantly higher in patients with postoperatively improved cognition than in those with postoperatively unchanged or impaired cognition, though the multivariate analysis did not demonstrate a significant difference. Considering the correlation between Cho/Cr ratios and cognitive functioning in the elderly population,18,21 the postoperative increase in Cho may imply recovery of abnormally reduced myelin membrane turnover, resulting in cognitive improvement after surgery.
The relationship between increases or decreases in cerebral metabolite and cognitive changes following CEA remains poorly defined. A recent study suggested that normalization of cerebral metabolism via improvement in cerebral hemodynamics after CEA may result in cognitive improvement.9 In contrast, cognitive impairment after CEA may result from various mechanisms.9 First, to perform CEA, the ICA and common carotid arteries are cross-clamped. There is a transient decrease in perfusion in the ipsilateral middle cerebral artery territory in some patients. When the reduction in hemispheric perfusion is significant enough to damage neuronal tissues, it may cause postoperative impairment of cognitive function accompanied by a reduction in cerebral metabolism. Second, a large percentage of patients exhibit evidence of gaseous and particulate emboli in the middle cerebral artery during CEA. The particulate embolization during surgery may result in decreases in cerebral metabolism and neuropsychological deterioration. Third, cerebral hyperperfusion after CEA is defined as a major increase in ipsilateral cerebral blood flow after surgical repair of carotid stenosis that is well above the metabolic demands of the brain tissue. This phenomenon often manifests with unilateral headache, face and eye pain, seizure, and focal symptoms that occur secondary to cerebral edema or intracerebral hemorrhage. Post-CEA hyperperfusion, even when asymptomatic, also causes postoperative cortical neural damage that results in postoperative cognitive impairment.
The univariate analyses in the present study showed that the ΔNAA/Cr ratio in the cerebral hemisphere contralateral to the operative site was significantly higher in patients with postoperatively improved cognition than in those with postoperatively unchanged or impaired cognition. However, multivariate analysis did not demonstrate the significance of this relationship. In the former patients, improvement in cerebral hemodynamics in the contralateral cerebral hemisphere after CEA may result in postoperative increases in cerebral metabolism. When there is contralateral ICA stenosis or occlusion in addition to ipsilateral ICA stenosis, perfusion in the contralateral cerebral hemisphere is often reduced before surgery and may be increased via collateral circulation from the ipsilateral ICA after surgery. However, the incidence of the contralateral ICA stenosis or occlusion was not different among the 3 subgroups of patients with different cognitive changes after surgery. Another possible mechanism may be related to improvement in brain perfusion in the contralateral anterior cerebral artery territory via the anterior communicating artery from the ipsilateral ICA after surgery when the A1 portion of the contralateral anterior cerebral artery is hypoplastic. However, this possibility was not investigated in the present study.
The present study has several limitations that require discussion. First, area ratios for NAA or choline were expressed as a ratio relative to creatine. The absolute value of each area can be obtained from MR spectroscopy. However, the absolute value includes errors arising from variations of magnetic field homogeneity, and converting to the ratio reduces such errors.35 Creatine concentration is relatively constant in each region of the brain, even in the context of metabolic disease or rapid fluctuation in energy metabolism.17,24 Muñoz Maniega et al33 demonstrated that while creatine concentration was significantly reduced in the ischemic cores, it did not change in surrounding tissue that otherwise appeared normal on MR imaging. Because the present study did not include patients with new postoperative ischemic lesions on MR imaging, postoperative changes of total creatine concentration may minimally affect our results. Second, the cerebral hemisphere ipsilateral to the ICA stenosis often exhibits brain atrophy even when massive cortical infarction is not detected on MR imaging in the cerebral hemisphere.36 In that situation, the proportion of CSF occupying the region of interest for measurement of cerebral metabolites by using MR spectroscopy is higher, thereby reducing the accuracy of metabolic ratios measured by MR spectroscopy.36 Finally, a single-voxel region of interest for measurement of metabolites was placed on the section through the centrum semiovale. The value in the single-voxel region of interest does not always reflect the metabolic condition in the whole cerebral hemisphere. Although a topographic map of cerebral metabolism can be obtained by using a multivoxel method, metabolic values acquired from a single voxel may provide more accurate quantification of cerebral metabolic ratios than those acquired from multiple voxels.11
Conclusions
The present study by using 3T proton MR spectroscopy in a relatively large number of patients demonstrated that postoperative changes in cerebral metabolites are associated with changes in cognitive function after CEA. Further investigations by using other modalities, such as oxygen 15 gas or [18F] fluorodeoxyglucose positron-emission tomography, would be of benefit to confirm the relationship between changes in cerebral metabolism and change in cognitive function after CEA.
Footnotes
Disclosures: Makoto Sasaki—UNRELATED: Consultancy: Hitachi Medical, GE Healthcare, Lundbeck, Actelion, Grants/Grants Pending: Ministry of Heath, Labor and Welfare of Japan.* Ministry of Education, Culture, Sports, Science and Technology of Japan,* Payment for Lectures (including service on Speakers Bureaus): Hitachi, GE Healthcare, Mitsubishi, Daiichi, Fuji, Otsuka, Sanofi, Johnson & Johnson, J-nac, Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Hitachi, GE Healthcare, Mitsubishi, Daiichi, Fuji, Otsuka, Sanofi, Johnson & Johnson, Olea. *Money paid to the institution.
This work was partly supported by a Grant-in-Aid for Strategic Medical Science Research Center of Ministry of Education, Culture, Sports, Science and Technology—Japan; and the Core Research for Evolutional Science and Technology of Japan Science and Technology Agency.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 8, 2012.
- Accepted after revision July 29, 2012.
- © 2013 by American Journal of Neuroradiology