Abstract
BACKGROUND AND PURPOSE: The risk of hemorrhage in the context of developmental venous anomaly is considered to be very low, but it has never been evaluated by susceptibility-weighted MR imaging at 3T. The goal of the present study was to evaluate the prevalence of hypointense foci (ie, microhemorrhage or cavernous malformation) associated with DVA on phase-sensitive MR imaging, on the basis of principles similar to those of susceptibility-weighted MR imaging, and to evaluate the relationship between the hypointense foci and several factors, such as white matter hyperintense lesions adjacent to DVA on T2-weighted imaging, DVA morphology, and clinical symptoms.
MATERIALS AND METHODS: This study retrospectively evaluated 61 lesions in 59 consecutive patients with DVA who underwent MR imaging including phase-sensitive MR imaging. Two neuroradiologists independently assessed for the presence of hypointense foci and other factors such as DVA location, depth, size, direction of draining vein on phase-sensitive MR imaging, and white matter hyperintense lesion on T2-weighted imaging. Clinical symptoms were also assessed.
RESULTS: Hypointense foci were observed in 62.3% (38/61) of lesions. White matter hyperintense lesion was more frequently observed in patients with hypointense foci (26/38) than in patients without hypointense foci (7/23) (P < .01). There was no significant association between hypointense foci and other factors.
CONCLUSIONS: Our results support the hypothesis that microhemorrhage or cavernous malformation can be related to venous congestion caused by abnormal venous drainage. We conclude that phase-sensitive MR imagingis useful for the detection of microhemorrhage or cavernous malformation in patients with DVA, especially when associated with white matter hyperintense lesion.
ABBREVIATIONS:
- CM
- cavernous malformation
- DVA
- developmental venous anomaly
- ICH
- intracranial hemorrhage
- PSI
- phase-sensitive MR imaging
- WMH
- white matter hyperintense lesion
Developmental venous anomaly, also called venous angioma, is the most common type of vascular malformation. The embryogenesis of DVAs is not well understood, but it is hypothesized that they result from a focal arrest of venous development and retention of primitive medullary veins.1 Mullan et al2,3 suggested that early occlusion of normal developing veins may lead to absence of normal cortical venous vasculature and compensatory formation of collateral dilated veins. Okudera et al4 concluded that DVAs arose from aplasia, hypoplasia, or occlusion of the various segments of the superficial or deep drainage medullary veins or distal pial vein immediately before opening into the dural sinus from the evaluation of microangiograms of postmortem-injected brain specimens.
Before the advent of MR imaging, DVAs were thought to be rare lesions that were associated with intracranial hemorrhage. However, the increasing use of MR imaging revealed that DVAs were relatively prevalent and were associated with a low risk of hemorrhage (reported prevalence of hematoma, 2.4–3.0%; ICH annual risk, 0.15–0.68%).5⇓⇓–8 MR imaging sometimes reveals hypointense foci that can be regarded as microhemorrhage or CM in the territory of the DVA. Several studies have characterized the prevalence of hemorrhage or CM associated with DVA.5⇓⇓⇓⇓–10 However, this phenomenon has not yet been assessed by susceptibility-weighted MR imaging at 3T. Thus, the first goal of this study was to assess the prevalence of hypointense foci by using phase-sensitive MR imaging on the basis of principles similar to those of susceptibility-weighted MR imaging at 3T.
Several types of brain parenchymal abnormalities within the drainage territory of DVA have been identified; these include WMH on T2-weighted imaging and FLAIR imaging, atrophy, and dystrophic calcification.7,11,12 WMHs sometimes coexist with hypointense foci. Although the pathologic correlation and etiology of WMH in the drainage territory of DVA remains unknown, it has been speculated that WMH reflects leukoaraiosis, which histopathologically includes edema, demyelination, and gliosis resulting from chronic venous hypertension caused by anomalous venous drainage.7,11 Meanwhile, the relationship between WMH and hypointense foci has not yet been studied. Therefore, the secondary goal of this study was to evaluate the relationship between hypointense foci and other factors, such as WMH.
Materials and Methods
Patient Population
We searched a computer data base of all radiologic results that were obtained at our hospital from January 2006 to December 2011 for MR imaging reports containing the terms “developmental venous anomaly” “venous angioma,” or “medullary venous malformation.” From these search results, patients who underwent MR imaging including PSI were selected. The criteria used to establish the diagnosis of DVA are based on MR imaging findings, especially on PSI. DVAs are characterized by a cluster of venous radicles that converge into a collecting vein, resulting in the typical caput medusae appearance. If the characteristic morphology is seen on MR imaging, DVA is strongly suspected. One patient was excluded because of susceptibility artifacts caused by postoperative changes around the DVA, and another 5 patients were excluded because the DVA region was not sufficiently included on PSI. One patient had 3 DVAs. Thus, 61 lesions in 59 consecutive patients (age range, 2–83 years; mean age, 54 years) were included in the final analysis.
This study was approved by the ethics committee of our university, and the requirement for written informed consent was waived because of the retrospective nature of this study.
MR Technique
All brain MR imaging was obtained with a 3T MR system (Signa Excite HD; GE Healthcare, Milwaukee, Wisconsin) by means of an 8-channel phased array coil.
Axial T2-weighted fast spin-echo images was obtained with the following imaging parameters: TR/TE, 4000/95 msec; 512 × 320 matrix, 21-cm field of view, and section thickness/intersection gap, 5/1.5 mm. For susceptibility-weighted MR imaging, PSI was performed with a 3D spoiled gradient-recalled acquisition in steady state sequence with flow compensation, by use of the following imaging parameters: TR/TE, 45/30 msec; flip angle, 20°; FOV, 21 cm; matrix, 512 × 192; section thickness, 1.5 mm; acquisition time, 7 minutes, 50 seconds. Phase-sensitive MR images were postprocessed by the use of a high-pass filter, and the images were converted into negative phase masks that were multiplied 4 times into the corresponding magnitude images by use of research software (PSIRecon: GE Yokogawa Medical Systems, Tokyo, Japan). A minimum intensity projection was used to display the processed data as PSI by use of contiguous 10.5-mm-thick sections with 7-mm overlap in the transverse plane (Advantage Workstation Version 4.1; GE HealthCare).
Image Reading
Two neuroradiologists (M.T., S.F.) who were blinded to the patient clinical information independently reviewed images on PSI and T2-weighted imaging. Assessments included the presence of hypointense foci around DVA on PSI and the presence of WMH in the drainage territory of the DVA on T2-weighted images. The drainage territory was defined as the brain parenchyma directly adjacent to the visualized radicles of the DVA. We also assessed the location of hypointense foci on PSI according to a previous study. Location of CM was classified into “central” or “peripheral,” according to whether the main location was a portion of the DVA where abnormal small tributaries of medullary veins gathered.13 Additionally, we evaluated DVA location, depth, and the size and direction of draining vein. The DVAs were classified by depth as juxtacortical, subcortical, and periventricular, according to previous studies.11,14 “Juxtacortical” (or superficial) was defined as within the gray matter or at the gray-white junction. “Subcortical” was defined as below the juxtacortical region but not adjacent to the ventricular wall. “Periventricular” (or deep) was defined as adjacent to the lateral, third, or fourth ventricle or within the center of the structure, such as the pons. The terminal or draining vein to which the caput medusae join was classified as either a deep (toward the ventricle) or superficial (toward the brain surface) draining vein. Concerning the distance of draining vein, the draining vein is “long” if the depth is juxtacortical and the direction is deep or if the depth is periventricular and the direction is superficial. The draining vein is “short” if the depth is juxtacortical and the direction is superficial or if the depth is periventricular and the direction is deep. All others are categorized as “median” draining vein.
Discordance between the 2 radiologists was resolved by consensus. To minimize bias, T2-weighted imaging assessments were performed 3 months after PSI assessments.
Furthermore, one neuroradiologist (M.T.) also assessed the presence of calcifications in patients with hypointense foci on CT images, which were performed within 1 year of MR imaging. Among 38 patients with hypointense foci, 14 patients underwent CT within 1 year from MR imaging.
Clinical and Imaging Finding Correlation
We retrospectively reviewed their clinical records, clinical indications for the examinations, and symptoms. Clinical indications and symptoms were tabulated for each case and assessed for correlation with the presence of hypointense foci.
Statistical Analysis
The relationship between hypointense foci and WMH, DVA location, depth, size and direction of draining vein, and the presence of symptoms was assessed by χ2 testing. A difference with a value of P < .05 was considered statistically significant.
Results
A total of 61 lesions in 59 patients with DVA were evaluated. Hypointense foci were observed on PSI in 62.3% (38/61 sides); WMH was observed on T2-weighted imaging in 54.1% (33/61 sides).
Table 1 shows the location of hypointense foci. The main location of hypointense foci was the peripheral portion of medullary veins.
Location of cavernous malformation
Table 2 outlines the presence or absence of WMH, DVA location, depth, and the size and direction of draining vein on the basis of hypointense foci. WMH was more frequently observed in DVAs with hypointense foci (68.4%) than in those without hypointense foci (30.4%) (P < .01) (Figs 1⇓–3). There was no significant association between hypointense foci and other factors.
Associations between hypointense foci and other factors
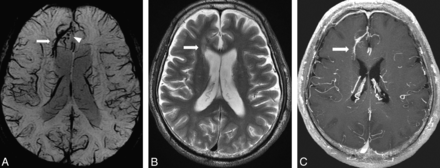
A 64-year-old man with DVA in the right frontal lobe. A, PSI shows DVA in the right frontal lobe (arrow) and hypointense foci around the DVA (arrowhead). B, T2-weighted image shows WMH around the DVA (arrow). C, DVA is enhanced by contrast agent administration, whereas hypointense foci are not enhanced on T1-weighted image.
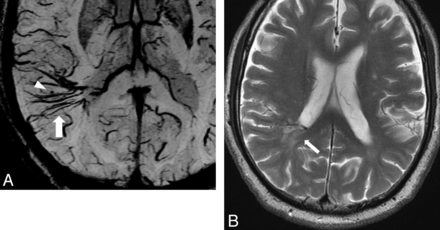
A 25-year-old woman with DVA in the right cerebellum. A, PSI shows DVA in the right cerebellum and hypointense foci around the medullary veins (arrowhead). B, T2-weighted image shows WMH around the DVA (arrowhead).
A 75-year-old man with DVA in the right parieto-temporal lobe. A, PSI shows DVA in the right parieto-temporal lobe (arrow) and minute hypointense foci around the DVA (arrowhead). B, T2-weighted image shows WMH around the DVA (arrow).
Table 3 outlines clinical indications and symptoms on the basis of hypointense foci. We evaluated the association between the presence of symptoms and hypointense foci. Although patients with hypointensities had higher rates of being symptomatic (71%) than those without (52%), no significant association was identified.
Patient symptoms and indications for examinations in the 2 groups based on hypointense foci
Among 38 patients with hypointense foci, 14 patients underwent CT within 1 year from MR imaging. Apparent calcification within the drainage territory of DVA was observed in 1 patient on CT images, but hypointense foci were also observed in other regions distant to the calcification.
Discussion
Our results demonstrated that the prevalence of hypointense foci, indicating microhemorrhage or CM, was higher (62.3%) than previously reported. According to previous studies, CM occurs in up to 18% of patients with DVA,7,9 whereas DVA is present in 8–33% of patients with CM.5,8⇓–10 The discrepancy in the prevalence of hemorrhage and CM between this study and previous studies may be related to several factors. First, susceptibility-weighted MR imaging was used in the present study but not in prior studies. Susceptibility-weighted MR imaging is a high-resolution 3D gradient-echo MR imaging technique with phase postprocessing that accentuates the paramagnetic properties of blood products, such as deoxyhemoglobin, intracellular methemoglobin, and hemosiderin. As a result, it is quite sensitive to the presence of even small amounts of hemorrhage. Previous studies have reported susceptibility-weighted MR imaging is more sensitive in detecting CM than T2*, which in turn is much better than T1 or T2.15,16 Susceptibility-weighted MR imaging has also been specifically recommended when imaging DVAs because of their association with CM.17 Second, 3T MR was used in the present study but not in prior studies, and the susceptibility effect in higher field strength increases the conspicuity of paramagnetic substances. Additionally, we could detect hypointense foci more frequently in the peripheral regions of medullary veins in comparison to a previous study that used T2-weighted imaging and T1-weighted spoiled gradient-recalled acquisition in steady state imaging with gadobutrol enhancement at 3T.13 This may result from the increased detectability of minute hemorrhage and CM in the peripheral region of medullary veins in response to the increased conspicuity of paramagnetic substances with PSI at 3T.
The incidence of WMH in the drainage territory of DVA in this study was >50%, whereas reported WMH rates on MR imaging were 12.5% and 28.3%.7,11 Although we have no good explanation for this discrepancy, variations in the imaging parameters and the scanners themselves could produce different results. For example, we used a 3T MR system, whereas both previous studies were mainly investigated by 1.5T MR systems. Moreover, the discrepancy may be due to the differences in the populations and readers among these reports.
This study also demonstrated a significant relationship between hypointense foci and WMH, which may reflect leukoaraiosis that histopathologically includes edema, demyelination, and gliosis resulting from chronic venous hypertension caused by anomalous venous drainage. That supposition is based on several reports of stenosis of the draining vein, which leads to chronic venous congestion,7,18,19 and on a report of reduced cerebral blood flow in the drainage territory of the DVA, which suggests the presence of altered hemodynamics.20⇓–22 Alternatively, some case reports have described de novo formation of CM in the drainage territory of DVA.23⇓–25 Although the exact events leading to de novo formation of CM in patients with DVA is unclear, some investigators have speculated that development of venous hypertension and resultant microhemorrhages from the fragile vessel wall of the DVA may induce reactive angiogenesis.26,27 Relative ischemia can result in production of angiogenic factors and stimulation of growth of new vessels.28 However, the neovasculature lacks vasoregulatory capacity, and its fragility makes it susceptible to bleeding. This leads to repeated hemorrhage and formation of abnormal vessels, which eventually results in CM formation.13,29 Hong et al13 reported that the angioarchitectural factors of DVA cause disturbances in blood flow and may lead to CM within the territory of DVA by increasing venous pressure. Taken together, these data suggest that venous congestion caused by angioarchitectural or other factors leads to WMH and subsequent CM formation. Hence, these 2 phenomena are likely to occur simultaneously.
In the present study, there was no significant association between the presence of hypointense foci and the presence of clinical symptoms. However, CM is generally considered to be an active lesion characterized by dynamic behaviors, including enlargement, regression, and de novo formation. Furthermore, several studies have reported that clinical presentation of patients with both DVA and CM is nearly always related to CM, reflecting its potential for epileptogenesis and symptomatic hemorrhage.5,9,29,30 Although no association was found between hypointensities and presence of clinical symptoms or indications, patients with hypointensities had higher rates of being symptomatic than those without (71% versus 52%) in the present study. This result shows a tendency that supports previous assumptions of CM being a dynamic lesion with the potential to cause bleeding and other symptoms. If analyzed with a larger population, there may be a significant association. Therefore, careful follow-up examination with the use of susceptibility-weighted MR imaging should be performed to assess whether there is a change in size of the hypointense foci, and further follow-up survey is needed to predict symptomatic ICH.
This study has several limitations. First, the study population was relatively small, and further studies in a large population are required to validate the present results. Second, hypointense foci may have represented dystrophic calcification or thrombosis rather than microhemorrhage and CM. However, only 1 among 14 patients with hypointense foci on PSI showed calcification on CT. Therefore, it is unlikely that calcification around the DVA plays a major role in the hypointensity on PSI, though minute calcification can coexist with hemorrhage.
Conclusions
The prevalence of hypointense foci, indicating microhemorrhage or CM, is higher than previously reported in patients with DVA. These hypointense foci may be related to WMH. PSI is useful for the detection of these hypointense foci in patients with DVA, especially when associated with WMH.
ACKNOWLEDGMENTS
We thank Eijirou Yamashita, PhD; Takuro Tanaka, BS; Nobuo Hashimoto, BS; and Shota Sakimoto, BS, for technical support in obtaining the high-quality MR images used in this study.
REFERENCES
- Received September 3, 2012.
- Accepted after revision November 1, 2012.
- © 2013 by American Journal of Neuroradiology