Abstract
BACKGROUND AND PURPOSE: Interlaminar LESIs are commonly used to treat LBP or radiculopathy. Most studies focus on the long-term outcomes of LESI. The purpose of this study is to evaluate the immediate effects of fluoroscopically guided LESI on LBP/radiculopathy including low- or high-strength anesthetic response.
MATERIALS AND METHODS: The procedure notes, post-procedure records, and imaging records dedicated spine nurse assessments, and imaging records were retrospectively evaluated in 392 fluoroscopically guided LESIs performed in 276 patients (nonrandomized, nonblinded; 131 males, 145 females; average age, 56 years) with LBP/radiculopathy using either low- or high-strength anesthetic (80 mg of methylprednisilone mixed with bupivacaine [0.25% or 0.5%]). Post-procedure documentation of the patient's pre- and postprocedure VAS pain-scale level were tabulated.
RESULTS: Single LESI was performed in 199 patients, with multiple LESIs in 77 (193 injections). Low-strength bupivacaine (0.25%) was used in 237 injections, with high-strength (0.5%) in 155. Complete to near-complete immediate pain relief (<20% residual pain) was reported after 197 of 392 (50.3%) injections. No pain relief was reported after 60 (15.4%) injections (>80% residual), with partial relief in the remainder. No statistical difference was noted between low- and high-anesthetic strength or between single- and multiple-injection patients. In multiple-LESI patients, consistent pain relief response was noted in 39 of 77 (50.6%) patients, with improving LESI response in 20.8%, deteriorating LESI response in 19.5%, and variable response in 9.1%.
CONCLUSIONS: An immediate pain-extinction response is identified after LESI, which appears independent of anesthetic strength. This observation may relate to pain origin and/or pain nociceptor afferent pathway in an individual patient and potentially relate to treatment response.
ABBREVIATIONS:
- AP
- anteroposterior
- DRG
- dorsal root ganglion
- LBP
- low back pain
- LESI
- lumbar epidural steroid injection
- VAS
- Visual Analog Scale
Interlaminar LESIs are commonly used for treatment of LBP or radiculopathy in patients with symptomatic lumbar degenerative disease, disk herniation, or spinal stenosis.1⇓⇓⇓⇓⇓⇓–8 Image-guided needle insertion is considered essential in confirming correct epidural placement of the injection.9,10 In most instances, steroid is mixed with either preservative-free local anesthetic or saline to enhance epidural spread of the injection.
Most studies evaluating the effect of LESI have focused on pain response weeks or months after injection.1⇓–3 Reported treatment response after LESI varies widely, which is probably related to the complex and potentially multifactorial spectrum of degenerative causes of spine pain being treated.
It has occasionally been noted that there is an immediate response to the combination of epidural steroid and local anesthetic after the injection, with some pain reduction.7,8 This study evaluates the immediate response to epidural steroid and anesthetic injection in a large cohort of patients, including the effects of low- and high-strength anesthetic.
Materials and Methods
Over a 6-year period, 435 consecutive LESIs of steroid comixed with local anesthetic were performed on 307 patients. In most of these patients, LBP was either the only symptom or the primary symptom, with a lesser complaint related to radiating leg discomfort. An LESI only was performed at these procedure encounters without additional spine injections. Our practice is primarily referred to by spine-focused neurosurgeons or orthopedic surgeons. Patients with clinically more well-defined causes of LBP or radiculopathy (ie, facet arthropathy, sacroiliac joint pain, disk herniation with root compression) are generally treated with focused injections (facet steroid, sacroiliac joint steroid, nerve root steroid).
Complete information with both pre- and postprocedure VAS pain data was available for 392 LESIs in 276 patients. The results of these 392 injections are the focus of this article. The procedure notes and radiology reports were retrospectively reviewed for the 276 patients included in the study. Information obtained included pain characteristics (LBP, radiating leg pain, both), recorded immediate preprocedure and immediate postprocedure VAS pain level (0–10 scale; 0 = no pain, 10 = worst imaginable pain), LESI location, and anesthetic concentration and volume used.
Incomplete information regarding pre- and postinjection pain levels was present in 43 injections. Twelve injections that were excluded (0.25% bupivacaine: 9 injections; 0.5% bupivacaine: 3 injections) were performed in 11 patients who had other LESIs included in the study. Thirty-one injections that were excluded were performed in 31 patients not included in the study but who had similar patient demographics (16 males, 15 females; average age, 54.2 years; symptom duration, 4.7 years [range 1 month to 40 years]; presenting symptoms: 7 LBP only, 23 LBP+leg, 1 leg only; 0.25% bupivacaine, 15 injections; 0.5% bupivacaine, 16 injections). Four additional patients had LESI for symptoms of spinal stenosis (neurogenic claudication only) and were therefore excluded. Institutional review board approval was obtained for this retrospective assessment.
The patients were initially assessed before the procedure by a dedicated spine intervention nurse, with documentation of basic clinical history, including description of the patient's back and/or leg pain characteristics and the specific immediate preprocedure VAS pain level.
Preprocedure imaging was reviewed before injection to assess for potential pain source and largest/safest posterior epidural space to access and for location of prior postoperative changes. LESI level was generally chosen as close as possible to the area of suspected abnormality. In the event of laminectomy/posterior epidural scarring, the adjacent superior level was usually selected.
LESIs were performed fluoroscopically by standard techniques. In the prone position, the back was cleansed, and the posterior epidural space just beneath the spinous process was targeted with slightly oblique AP fluoroscopy and local anesthetic was applied. A 20-ga Tuohy needle was introduced and progressively advanced to the posterior epidural space. Confirmation of needle-tip position was made with fluoroscopy, loss of pressure resistance, and a small 1- to 1.5-mL injection of nonionic contrast material (iohexol 240). After confirmation of needle-tip position, a mixture of 80 mg of methylprednisilone acetate (1 mL), comixed with either low concentration anesthetic (0.25% bupivacaine, average 1.98 mL/injection; range 1.5–2.5 mL) or high concentration anesthetic (0.5% bupivacaine, average 2.02 mL/injection; range 1.5–2.5 mL), was injected. Choice of anesthetic in any given patient or injection was based on anesthetic material availability and/or injection location relative to the conus, without specific control, specific randomization approach, or relationship to prior injections.
Injection volume was generally based on patient body habitus and spinal canal size, along with injection location, relative to the conus for concern of potential conus anesthesia. A slightly larger injection volume of anesthetic (2–2.5 mL) was used in normal/larger patients, and a slightly smaller volume of anesthetic (1.5 mL) was used in smaller patients, when injecting close to the conus or when injecting in the area of severe multilevel spinal stenosis, as identified on preprocedure MR imaging. Free dilution and spread of the epidural contrast when injecting the anesthetic/steroid mixture was visualized fluoroscopically, confirming epidural anesthetic/steroid delivery, and documented in the AP projection in all patients.
After the procedure, the patient was questioned about pain while still in the procedure suite. The patient was then taken to the spine assessment area by the nurse, where postprocedure assessment, including the postprocedure VAS level of the primary pain complaint, was performed just before discharge (10 minutes postinjection).
Residual pain percent (%) was calculated and tabulated as postprocedure VAS/preprocedure VAS × 100.
Statistical Analysis
The percentage of pain remaining postinjection was categorized as either 0%, >0% and <20%, ≥20% and <40%, ≥40% and <60%, ≥60% and <80%, ≥80% and <100%, or 100%. The proportion of patients achieving at least 30% reduction in pain severity was also calculated. Multinomial regression was used to examine the associations between concentration of injection (0.25% bupivacaine, 0.50% bupivacaine) and percent of pain remaining, and between injection events (multi, single) and percent of pain remaining, respectively. Responses from different subjects are assumed to be statistically independent, and responses within subjects (as in the case of multievents) are assumed to be correlated; hence, the need to allow for repeated measures. PROC GENMOD permits repeated measures and was used for the analysis. SAS software was used for all analyses (SAS Institute, Cary, North Carolina). Statistical significance was set at P < .05.
Results
Of the 276 patients, 145 were female and 131 were male, with an average age of 56 years (range 16–87 years). LBP was the primary clinical complaint in 264 patients (LBP only, 78 patients; LBP with variable but lesser degree of buttock/gluteal/leg pain, 186 patients), with leg pain only in 12 patients. Forty-two patients had prior surgery.
A single LESI was performed in 199 patients (72%), with 77 patients (28%) having multiple LESIs (193 injections). Most (62.8%) injections were performed at L3–L4 (LESI locations: T12–L1, 1; L1–L2, 25; L2–L3, 78; L3–L4, 246; L4–L5, 38; L5–S1, 4). Procedure-related complications were encountered in only 2 patients. All injections were epidural in location, as evidenced by free flow of contrast in the epidural space at fluoroscopy. In 1 patient, dural access was encountered initially, but successful LESI was ultimately performed at a cephalad level. A second patient had severe multilevel spinal stenosis, with successful LESI in the middle of the stenotic region; transient paraparesis occurred postinjection, probably related to migration of the anesthetic bolus to the level of the conus.
The overall postprocedure pain injection results are summarized in Table 1. Complete to near-complete pain elimination (residual pain: 0 to <20% of initial VAS score) occurred after 197 of 392 (50.3%) injections (complete pain elimination: 172 [43.9%]). Complete relief was surprisingly recognized by the patient immediately upon standing after 28 LESIs. Variable immediate pain improvement was noted in the remaining 195 injections. Negligible pain reduction (80%–100% of initial VAS score) was noted after 60 of the 392 (15.4%) injections, with 10 patients reporting minor pain improvement and 50 (12.8%) reporting no change in their preprocedure pain. The proportion of patients achieving at least 30% reduction in pain severity was similar in low- and high-anesthetic-concentration groups (low anesthetic concentration: multiple, 77.6%; single, 74.1%; all low, 75.9%; high-anesthetic-concentration: multiple, 85.3%; single, 79.3%; all high, 81.9%; all injections: multiple, 80.3%; single, 76.4%; all injections, 78.3%). No significant difference was noted between patients who had either a single injection or multiple LESI procedures (P = .12). No difference in pain response was noted relative to injection location. Average preprocedure VAS pain score was 5.8; average postprocedure VAS pain score was 1.9. Two patients reported mild bilateral leg numbness after the injection but no motor weakness. Both were discharged without incident.
Injection response, single and multiple injection groups
Low-anesthetic-concentration LESI (0.25% bupivacaine) was performed in 237 injections; high-anesthetic-concentration LESI (0.5% bupivacaine) performed in 155 injections. These results are summarized in Table 2. Demographic characteristics of low-anesthetic-concentration and high-anesthetic-concentration groups are summarized in Table 3. Average preprocedure and postprocedure VAS pain scores were equivalent in low- and high-concentration-anesthetic groups (Table 3). No significant difference was noted in the percentage of immediate pain reduction recorded just before discharge in either the low-anesthetic-concentration or high-anesthetic-concentration groups (P = .70). In each group, approximately 50% of patients experienced complete or near-complete relief (0 to <20% residual pain) after the procedure. The average volume of anesthetic injected was essentially equivalent in both low- and high-anesthetic-concentration groups (Table 3).
Injection response in low-dose (0.25%) and high-dose (0.5%) bupivacaine groups
Demographic features of low- and high-concentration-anesthetic groups
Multiple Injections
The results for the 77 patients having multiple injections are further summarized in On-line Tables 1–4. A consistent immediate response to the LESI procedure was noted in 39 of 77 patients (50.6%; On-line Table 1; Fig 1). Progressive improvement in the immediate LESI response was reported in 16 of 77 patients (20.8%; On-line Table 2), with progressive deterioration of the immediate LESI response in 15 of 77 patients (19.5%; On-line Table 3). A variable response with different degrees of immediate improvement with each injection was noted in 7 of 77 of patients (9.1%; On-line Table 4). Distribution of initial injected contrast and characteristics of contrast dilution appeared similar or identical between initial and repeat injections in the multiple-injection patients. No substantial change or diminution of dilution or epidural spread was apparent with subsequent or later injections.
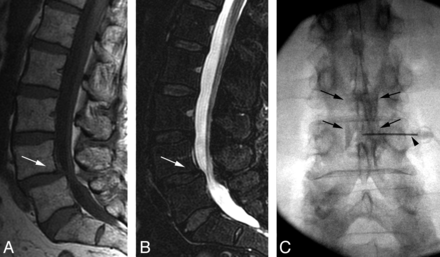
Patient is a 65-year-old woman with LBP and slight radiating right leg pain (VAS 7/10). LESI was performed ×4. After each injection, her pain dropped to VAS 0/10. A and B, Sagittal T1- and T2-weighted images of the lumbar spine demonstrate degenerative spondylolysthesis at L4–L5 (arrows). C, AP fluoroscopic image after injection of steroid and anesthetic mixture (0.5% bupivacaine), with Tuohy needle position at L3–L4 (arrowhead) demonstrates dispersal of the test contrast material in the epidural space in a typical fashion (arrows). All 4 injections demonstrated a similar contrast dispersion pattern.
Discussion
Image-guided injection procedures are commonly used to diagnose or treat spine-related pain, including the facet, sacroiliac joint, exiting nerve root, and the disk.11,12 The anesthetic injection, either alone or in combination with steroid, serves as a diagnostic block, and elimination of the patient's pain is generally interpreted as accurate targeting of the pain generator. For most injection procedures, the long-term pain response is referenced to the clinical diagnosis, in part related to history or physical examination, but to a large extent related to the confirmatory nature of the pain response to the diagnostic block.
LESI is a more generic injection often used for both specific symptoms (disk herniation with radiculopathy, stenosis, lateral recess impingement) as well as the challenging problems of nonspecific or multifocal LBP, where targeting is less specific due to the complex morphologic imaging appearance and clinical symptoms. Steroid is typically mixed with preservative-free local anesthetic or saline, but the volume used varies widely. In some instances, more specific targeting of the region of pain is attempted; in other instances, larger injection volumes are used to help apply the steroid diffusely in the spinal canal. An early anesthetic response from the LESI has been recognized by several authors but has not been focused on, probably due to nonspecific LBP or radiating leg pain present in so many instances.7,8
Our data demonstrate complete or near-complete extinction of the patient's pain in 50% of patients after the LESI, with partial or no pain improvement in the remainder. In patients having multiple injections, 50% demonstrate a consistent and reproducible response, suggesting a specific and definable mechanism of action. In addition, this response appears to be independent of the 2 local anesthetic concentrations we injected. The mechanism behind the immediate response to the LESI anesthetic-steroid injection is as yet not clear, but this response may have important implications in diagnosing the causes of LBP and predictive value for LBP treatment response.
The causes of LBP and radiating leg pain/sciatica are complex. Herniated nuclear material is known to have a significant acute inflammatory effect on epidural tissues, nerve roots, and the DRG.13⇓–15 Inflammatory mediators such as phospholipase A2, tumor necrosis factor-α, interleukin-6, interleukin-8, and prostaglandin E2 have been found in degenerative and herniated disk material.15⇓⇓⇓⇓⇓–21 Increased intradiskal cytokines have been demonstrated in patients with diskogenic low back pain as well as sciatica.20 Direct action of nucleus pulposus on the epidural space, nerve root, or DRG have demonstrated histologic changes (edema, hemorrhage, thrombus, nerve fiber injury, hyperemia) and nerve function changes (pain sensitivity, altered conduction velocity, spontaneous discharges, altered blood flow, increased endoneural pressure).22⇓–24 Direct action of selected inflammatory mediators and cytokines has also been demonstrated.25⇓–27
Acute nerve root or DRG compression can also induce inflammatory changes with alteration in nerve function.28,29 The DRG is sensitive to compression, leading to a marked increase in nerve discharge rate. Altered intraneural and perineural permeability occurs with the development of edema, increased intraneural pressure, and decreased neural blood flow. Ischemic and compressive nerve fiber injury occurs and long-term intraneural fibrosis can develop. With more chronic compression, inflammatory cell infiltration, edema, and intraneural fibrotic changes are recognized.30⇓–32 A combination of compression and inflammatory change has been shown by several groups to be synergistic.33,34
Potential Direct LESI Action on the Responsible Pain Source (LESI “Targeting Effect”)
Sustained, long-term pain improvement after LESI is thought to be related to the anti-inflammatory effects of the injected steroid on symptomatic structures in the epidural space, including the disk, dura, peridural tissues, and neural structures. Most patients who respond to LESI report pain relief beginning several days after the injection, presumably when the anti-inflammatory action becomes recognizable. The injected steroid has several putative actions on inflamed, sensitive tissue, including stabilization of cellular membranes, with reduced tissue and neural edema; a direct anti-inflammatory effect; inhibition of neural peptide synthesis/action; inhibition of prostaglandin synthesis; suppression of neuronal inflammatory discharges; suppression of sensitized dorsal horn neurons; and alteration of neuronal blood flow.34⇓⇓⇓⇓⇓⇓⇓–42 There is also a potential anti-inflammatory effect related to the injected local anesthetic that could complement or augment the steroid effect.38,43⇓–45 Recently, interferon-γ has been demonstrated in epidural lavage samples, and LESI response may equate to the level of interferon-γ reduction.46,47
The immediate LESI response could be related to direct local anesthetic action on the active epidural, neural, or perineural pain generator(s) or their immediate neural supply. If the patient's pain is related to irritation from inflammatory by-products (ie, disk) or inflammation of the epidural structures (dura, annulus, epidural vessels, epidural fat), the injected local anesthetic might be affecting these sensitized regions directly, causing immediate pain reduction. The direct response could also be amplified by potential dilution or limited “washout” of the locally active epidural inflammatory mediators.48 Alternatively, if pain is related to structures adjacent to the epidural space, direct anesthetic applied to the structure surface or nerve supply (ie, disk via sinuvertebral nerve) might be extinguishing the pain.
The immediate LESI response could therefore be a “targeting” indicator of the location responsible for the patient's pain, potentially serving as a predictor of a sustained LESI treatment response.
Potential LESI Action on Sympathetic Nociceptors (LESI “Signal Transmission Effect”)
Spinal or epidural anesthesia is routinely used for major surgical procedures of the lower body and extremities.49 Blockade effectiveness depends upon the local anesthetic used (amide/ester, pKa, lipid solubility), along with characteristics of the nerve fibers being anesthetized (myelination, fiber diameter).49,50 A differential sensory block is known to occur at the upper margin of the epidural anesthetic block, due to differing sensitivity of nerve fibers with unmyelinated C fibers blocked first, followed by myelinated A fibers.49,51⇓–53 At the upper margin of the spinal anesthetic, due to differential fiber sensitivity to the anesthetic, there is a separation between sensation to temperature, pain, and light touch.
This differential blockade effect has been previously explored using low-dose or dilute anesthetic, and has been suggested as a testing method that can help differentiate sympathetic from somatic lumbar pain-fiber contributors.54 Sympathetic pain fibers tend to be small unmyelinated C fibers. It is possible, therefore, that, in a similar fashion, the small-volume dilute anesthetic used in the LESI is acting as a low-dose or dilute blockade. Small-volume, locally delivered epidural anesthetic injection has been shown to retain a local regional block effect.55
Cortical somatosensory evoked responses are retained with lumbar epidural blockades, with neural pathways remaining intact, even in the setting of adequate surgical anesthesia.56 No significant difference in cortical evoked response is noted between control and 10 mL of 0.25% bupivacaine, but a concentration-related effect on evoked potential amplitude is found with 10 mL of 0.5% and 10 mL of 0.75% bupivacaine.57
The immediate LESI response and lack of a “dose-related effect” to anesthetic concentration, as found in our data, might suggest that an anesthetic effect on afferent sympathetic pain fibers is occurring, similar to selective sympathetic blockade.
Complete versus Absent versus Partial LESI Immediate Improvement
Complete or near-complete pain extinction was noted in 50% of injections immediately after LESI, with the remainder demonstrating variable pain improvement. Complete immediate pain improvement after LESI could be related to either comprehensive direct local anesthetic action on the painful region (LESI “target effect”) or comprehensive direct anesthetic action on ascending afferent sympathetic nociceptor pathways (LESI “signal transmission effect”).
In patients with no immediate pain improvement after LESI, the location of pain and/or local nociceptors might not come in direct contact with the anesthetic-steroid mixture (ie, granulation tissue) and therefore no immediate pain improvement occurs. Separately, the pain might be relayed via paraspinal sympathetic fibers that are entering the spinal canal or spinal cord more cephalad (ie, T11, T12) and is thus not in continuity with the epidural anesthetic.
In patients with a partial response, 2 or more pain generators might be present, 1 more sensitive and 1 less sensitive to the epidural anesthetic. The pain generator(s) might have mixed somatic and sympathetic nociceptor innervation with variable sensitivity to the epidural anesthetic. A combination of multiple pain generators and mixed somatic/sympathetic nociceptor innervation might be present, leading to a partial immediate response. Last, anesthetic contact time could play a role, and partial responses may reflect partial anesthetic contact with the locally painful site.
Consistent versus Variable LESI Response
In patients with multiple LESI procedures, both consistent and variable immediate-pain-response patterns were noted. Consistent immediate pain response (50% of patients with multiple injections) suggests a stable pain problem from LESI-sensitive pain generator, related to direct action on the pain generator or consistent sympathetic signal-ablation effect. Variable immediate responses (remaining 50% of patients with multiple injections [progressive improvement: 20.7%, worsening response: 19.5%, variable response 9.1%]) could be related to a number of factors.
Patients obtain LESI to return to a more normal life. Renewed normal or stressful activity might reaggravate a pain generator or aggravate a new area. A differing follow-up immediate response could reflect pain treatment effect and/or the presence of more than 1 pain generator.
An improving response could suggest successful steroid treatment of 1 pain generator, with the remaining generator simply more sensitive to the anesthetic injected, or it could reflect the presence of a new pain generator. A worsening immediate response could suggest the opposite, with improvement in the pain generator that was sensitive to the anesthetic and the remaining or new-pain generator being less sensitive to the epidural anesthetic. A variable response could represent a mixture of these.
Another potential cause of variable response to the LESI could be distribution of the injection. Although LESIs were performed in a standard fashion, control over LESI distribution is limited. The epidural space is complex, with defined but variably positioned meningovertebral ligaments between the thecal sac and the osteofibrous walls of the spinal canal.58 This can affect LESI flow and ultimately potentially affect anesthetic/steroid distribution.59,60 As stated here, the observed contrast dilution appears consistent when assessing multiple injections in the same patient (Fig 1), supporting reproducibility of the immediate LESI response. Confirming the exact distribution of the anesthetic/steroid mixture after each injection could be helpful, but might require a more specific technique approach.
As evidenced in 50% of our patients with multiple injections, though, a moderate degree of reproducibility can be demonstrated.
Limitations
While comparing VAS pain scores is an established measure of symptom change,61 preprocedure cognitive/psychologic factors (ie, depression, anxiety, catastrophizing) and other factors related to the patient's postprocedure function level have not been evaluated but might be important. Our population, while standard for LESI, is weighted toward LBP, and our pooled results might not apply to specific subgroups (acute versus chronic radiculopathy from disk protrusion/lateral recess impingement; LBP from acute disk herniation). Duration of the effect was not tested and might be important to understand.
While difficult to quantify, as well as controversial, another potential contributor to the immediate response to the LESI is the “placebo effect.” Data on epidural injection of saline is limited. Epidural saline injection has been compared with epidural lidocaine in detection of cutaneous current perception threshold, with positive response to lidocaine but no effect with epidural saline alone.62 In comparisons of postoperative pain control using top-off epidural lidocaine anesthetic or epidural clonidine, epidural saline had a negligible effect on postoperative pain, with clear response to epidural lidocaine or clonidine injection.63⇓–65 Alternatively, when analyzing somatosensory evoked potentials, decrease in amplitude is demonstrated with epidural lignocaine only, but increasing signal latency is demonstrated with both epidural lignocaine and saline, potentially related to a cooling effect.66 Washout effect can also occur with epidural saline, diluting local inflammatory mediators which either independently act on epidural nociceptors or a contribution to the epidural anesthetic effect.67 In addition, partial sympathetic blockade has been demonstrated with intrathecal saline.68 Therefore, progressively reduced epidural anesthetic concentration, or even epidural saline alone, could have a specific effect on pain perception. To this end, the exact action of epidural “placebo” is not known, and the most appropriate choice of placebo or sham comparison with epidural anesthetic is recognized as complex and controversial.67,68
Conclusions
An immediate pain-extinction response to LESI using anesthetic and steroid exists. The effect appears independent of local anesthetic concentrations used. Complete or near-complete immediate extinction of LBP is observed in approximately 50% of injections, with the remainder demonstrating a lesser response.
In patients having multiple LESIs, a consistent injection response is observed in 50%. Differing responses to follow-up injections were also found, with improvement in immediate pain response in 21%, worsening in 20%, and variable response over multiple injections in 9%.
The immediate pain response could have diagnostic implications, as well as treatment predictive value, and represent a powerful tool for patient selection or treatment planning.
Footnotes
Disclosures: Rex Jennings—RELATED: Support for Travel to Meetings for the Study or Other Purposes: UPMC reimbursement for presentation, Comments: Standard reimbursement for presenting some of the preliminary results at the ASSR convention in 2006 contained in the current paper.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- Received January 13, 2012.
- Accepted after revision March 28, 2012.
- © 2013 by American Journal of Neuroradiology