Abstract
BACKGROUND AND PURPOSE: Because alteplase does not penetrate thrombus effectively, this study examined whether a method thought to maximize surface distribution of alteplase on the offending thrombus during IATT would result in greater reperfusion rates in acute ischemic stroke.
MATERIALS AND METHODS: Clinical information, arteriograms, and CT scans following treatment from 85 consecutive patients who underwent IATT by using alteplase within 6 hours of stroke symptom onset were reviewed. Alteplase was delivered through a microcatheter embedded within the thrombus at 1 mg per minute in all cases, and the delivery never exceeded 100 mg of alteplase. Patients who underwent microcatheter contrast injections confirming that alteplase surrounded the thrombus were compared with patients who did not.
RESULTS: Greater than 50% vascular territory reperfusion occurred in 82.2% of patients who underwent IATT with the intention of optimizing alteplase delivery versus 30.0% in patients without this intention (P < .0001, Pearson correlation) with an odds ratio of 15.8 based on nominal regression analysis. Hemorrhagic complication rates between methods were similar. The mRS at 1–3 months, infarct volume, change in NIHSS score by 24 hours, and hospital discharge were positively affected by optimizing alteplase delivery.
CONCLUSIONS: A method that intends to evenly distribute alteplase around a thrombus resulted in better reperfusion rates and clinical outcomes compared with methods without this intention. Other predictors positively influencing reperfusion included the presence of slow antegrade flow distal to the clot, earlier time to treatment, lower presenting NIHSS score, and proximal occlusion site.
ABBREVIATIONS:
- IA
- intra-arterial
- IATT
- intra-arterial thrombolytic treatment
- mRS
- modified Rankin Scale
- OAD
- optimized alteplase delivery surrounding the thrombus
- RMP
- random microcatheter placement within the thrombus for alteplase delivery
- SAF
- slow antegrade opacification of contrast distal to the occlusion site
- TICI
- Thrombolysis in Cerebral Infarction
IATT has the potential to recanalize and reperfuse occluded cerebral vessels in the setting of an acute ischemic stroke. This, in turn, can rescue brain within an ischemic penumbra, which is characterized by delayed tissue perfusion surrounding already infarcted tissue, increasing the chance for significant clinical improvement. However, methods of recanalization by alteplase infusion in acute ischemic stroke have not previously been compared, to our knowledge. A technique that can maximize the effectiveness of thrombolytic therapy can be valuable. In vitro models have found that alteplase binds strongly to the fibrin matrix, unlike other forms of rtPA causing it to collect mainly on the surface of a thrombus.1 As a result, larger occlusive thrombi do not effectively respond to circulating alteplase because only a small proportion of the clot is being actively degraded. When a larger surface area of the clot is exposed to alteplase from slow antegrade blood flow around the clot, it has been shown that higher recanalization rates result.2 Methods that increase the distribution of alteplase surrounding the thrombus may improve recanalization rates. The goal of this study was to determine whether a method that achieves a more homogeneous distribution of alteplase throughout the surface of the occlusive thrombus would result in greater reperfusion rates compared with methods that do not.
Materials and Methods
This study was approved by the local institutional review committee. Data were collected from the review of consecutive patients who underwent IATT with alteplase at a single institution. Patients who received IV tPA or bridging IV/IA tPA therapy were not included in this study. Patients seen within 6 hours of symptoms of a carotid or within 12 hours of a vertebrobasilar stroke who were considered thrombolytic candidates by using clinical, laboratory, CT, and angiographic criteria derived from the Prolyse in Acute Cerebral Thromboembolism study underwent screening cerebral angiography.3,4 Unlike the Prolyse in Acute Cerebral Thromboembolism study, this study also included patients with ischemic stroke involving territories other than the MCA, and patients older than of 85 years of age. All patients seen between 0 and 3 hours were offered IV alteplase as an alternative to IA alteplase. Patients underwent angiography and thrombolytic treatment by using local anesthesia, an aseptic technique, and a biplane angiographic suite. Sedation was avoided when possible.
Presentation NIHSS score, NIHSS score 24–36 hours following ictus, NIHSS score at discharge, mRS score at 1–3 months, presentation laboratory values (platelet count, glucose level), systolic blood pressure, length of hospital stay, time to treatment, age, and sex were all recorded prospectively. Change in NIHSS score from the time of ictus to 24–36 hours following ictus and change in NIHSS score from the time of ictus to time of hospital discharge were calculated. NIHSS score and mRS were determined by a stroke neurologist (A.S., Y.M.). Symptomatic hemorrhagic transformation was defined as any hemorrhagic transformation associated with an increase in NIHSS score from the time of ictus to 24–36 hours following ictus by ≥4. Infarcted regions and foci of hemorrhage were traced out on axial cross-sectional images by a Certificate of Added Qualification−certified neuroradiologist (G.A.C), and areas were calculated by using Impax image analysis software (Agfa Corporation, Ridgefield Park, New Jersey). Infarct and hemorrhage volumes were then determined by multiplying cross-sectional areas by section thickness and adding them. A large hemorrhage was defined as one >25-mL volume.5
Angiographic evaluation included occlusion site, assessment of pial collaterals, SAF, and assessment of the results of treatment (extent of reperfusion). The site of occlusion was assessed on the anatomic site of occlusion as either proximal or distal if visible on angiography. Occlusions were considered proximal if the site of occlusion was at the internal carotid artery, basilar artery, or a proximal cerebral occlusion (M1, A1, or P1) segment. Occlusions were considered distal if the occlusion was at or more distal to the M2, A2, or P2 cerebral arterial segments or involved arterial branches. Reperfusion was assessed as a percentage of the affected vascular territory that was revascularized. The number of equivalent branches within the involved territory that reperfused was counted and divided by the number of expected branches. TICI reperfusion scores were then assigned as follows: A TICI score of 0 was given with no reperfusion to the affected territory; a score of 3 was given with complete reperfusion and recanalization of the affected territory. A score of 1 was given with reperfusion of <50% of the affected territory, and a score of 2 was given with reperfusion of ≥50% of the affected territory. Reperfusion involving <50% of the expected branches in the vascular territory involved was considered poor reperfusion. In this study, complete reperfusion was considered to be complete recanalization with complete reperfusion.6 Slow flow within a distal vessel following reperfusion with no angiographically visible occlusion was considered to represent reperfusion. Pial collaterals were graded as good or poor on the basis of the anatomic extent as defined elsewhere.7 Good pial collaterals were equivalent to grades 3 and 4 as described by Higashida and Furlan.8
Alteplase was delivered at 1 mg per minute in all cases and never exceeded 100 mg. All patients receiving alteplase were not anticoagulated following treatment and had involvement of 1 major vascular territory. If infusion was begun before 6 hours but the patient did not recanalize by 6 hours, continued infusion of tPA was left to the discretion of the physicians involved. The following are methods for alteplase delivery:
1) RMP within the offending thrombus followed by continuous infusion of alteplase. Microwire and microcatheter manipulation within the clot during tPA delivery was routinely performed. Use of this methodology was confirmed by the review of reports and discussions with the operators. Three different operators used this methodology. Although with this method the microcatheter was confirmed to be embedded in the thrombus, this method did not evaluate the distribution of contrast around the clot before alteplase delivery.
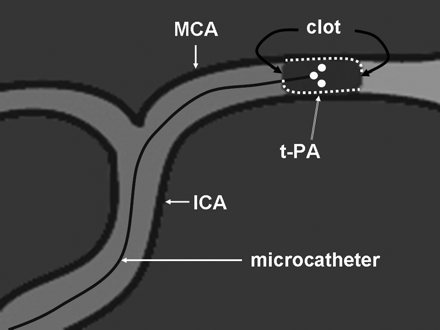
2) Microcatheter placement within the offending thrombus with periodic gentle microcatheter contrast injections and microcatheter repositioning to ensure that alteplase delivery surrounded the thrombus throughout the treatment (Figs 1⇓–3). Use of this OAD methodology was confirmed on the basis of review of radiology reports and discussions with the operator. Only 1 operator used this methodology. The OAD method is distinct from RMP because the microcatheter was meticulously repositioned throughout the procedure in a manner indicating that alteplase was continuously delivered around the clot as depicted in Fig 2. Figure 3 depicts how drug delivery can differ depending on the exact position within the offending thrombus. The ideal position would be one in which the thrombus is maximally exposed to alteplase. With the RMP method, any microcatheter position would be acceptable as long as it was positioned in the thrombus.
Optimizing microcatheter position within a thrombus with gentle alteplase infusion can help ensure that alteplase (dots) surrounds the thrombus during treatment.
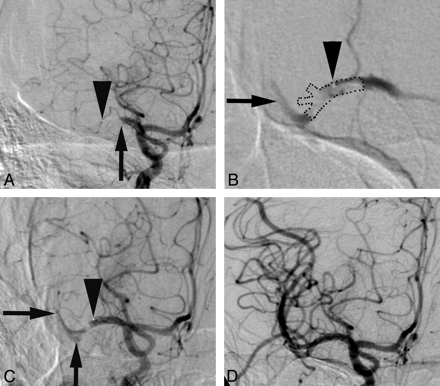
Arteriographic images were obtained before (A), early during alteplase infusion (B), late during alteplase infusion (C), and following alteplase infusion (D) in a patient with occlusion at the proximal M1 segment (arrow, A). The microcatheter tip is indicated by arrowheads. The microcatheter was initially positioned within the offending thrombus, and contrast injection suggests the expected distribution of the alteplase surrounding the thrombus (thrombus is outlined by dots, B). Contrast is identified in 1 major division branch distal to the thrombus (arrow, B) and proximal to the thrombus. Eventually thrombus begins to dissolve (C), and the microcatheter is repositioned to distribute alteplase throughout the thrombus in other divisions of the M1 segment (arrows, C). The final arteriogram demonstrates complete reperfusion and identification of 3 major branches originating from the M1 segment at the site of the thrombus.
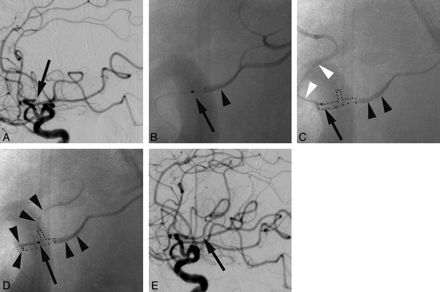
Arteriograms obtained before (A), during microcathter positioning (B−D), and following alteplase infusion (E) in a patient with occlusion at the superior division of the middle cerebral artery at the M2 segment (arrow, A) demonstrate optimization of microcatheter placement. The microcatheter tip is indicated by arrows (B−D). Arrowheads (B−D) indicate contrast distribution during microcatheter contrast injection. B, The microcatheter tip (black arrow) is located along the distal aspect of the thrombus. Contrast administration in B indicates that given this position, thrombolytic agent would only be distributed within the distal aspect of the thrombus. Contrast injection in C indicates that the thrombolytic agent is delivered to the proximal and inferior aspect of the thrombus with significant amounts of alteplase escaping into vessels proximal to the occlusion site (white arrowheads). Contrast injection in D indicates that thrombolytics are delivered around the entire thrombus (outlined by dots) and serves as an optimal position for thrombolytic infusion. Note that contrast enters 2 involved branches in D, unlike in B or C. Involved branches will demonstrate stagnant contrast opacification, whereas uninvolved branches will demonstrate brisk flow of contrast. It is intuitive that stagnant contrast would also imply that alteplase would also tend to stagnate adjacent to the thrombus during its delivery. If the RMP method was used all 3 microcatheter positions depicted in B−D would be acceptable, whereas with the OAD method, only the position in D would be acceptable.
The primary outcome measure for this study was reperfusion. Nominal regression analysis for good reperfusion (>50% reperfusion) was performed. Predictors tested were dichotomized for the purpose of this analysis and included time to treatment dichotomized at 270 minutes, site of occlusion (distal versus proximal), SAF before treatment,2 history of diabetes, glucose level dichotomized at 140 mg/dL, presenting NIHSS score dichotomized at <16 and ≥16, sex, age older than and equal to or younger than 60 years, anterior-versus-posterior circulation infarction, platelet level dichotomized at 200, and microcatheter technique. All factors with P < .10 were entered into the final model by using backward selection.
Secondary outcome measures included the rates of change in NIHSS score from the time of ictus to 24–36 hours following ictus and change in NIHSS score from the time of ictus to time of hospital discharge >4, the rate of mRS score at 3 months ≤2, mortality by any cause at 90 days, the rate of infarct volumes <50 mL, the rate of hemorrhagic transformation, the rate of symptomatic hemorrhagic transformation, and the rate of significant hemorrhage volume (>25 mL).
Results
A total of 85 patients, who fulfilled entry criteria, underwent IATT between April 1999 and April 2007. Presenting variables studied did not differ significantly when comparing methodologies for alteplase delivery (Table 1). Intraobserver agreement for this scoring method for pial collateral formation was identical in 87% of patients and differed by 1 scoring point in the rest (κ = 0.81, standard error = 0.065). The OAD methodology resulted in significantly higher reperfusion rates but not statistically significant different hemorrhagic complication rates (Tables 2⇓–4). Logistic regression analysis identified additional factors that may contribute to improved reperfusion. Reperfusion of ≥50% of the territory occurred with the presence versus the absence of each of the following factors: presence of slow antegrade flow distal to the offending thrombus (90.0% versus 46.8%), time to treatment <270 minutes (74.4% versus 43.5%), presenting NIHSS score <16 (66.7% versus 51.0%), and proximal occlusion site (60.6% versus 47.4%). In 16 patients, treatment was started within 180 minutes of symptom onset (10 with the RMP method and 6 with the OAD method).
Presenting clinical information and angiographic data dichotomized by the method of recanalization
Thrombolytics were curtailed early with only TICI 0 or TICI 1 reperfusion in 18 patients who underwent IATT by using the RMP method and 5 patients with the OAD method. Using the RMP method, the operator chose to limit infusion in 8 patients because time to treatment was close to the 6-hour limit (dose range was 10–40 mg of alteplase), in 4 patients because the operator chose to limit infusion in distal vessels (dose limited to 20 mg in all 4), in 2 patients who became hemodynamically unstable (dose limited to 20 mg and 35 mg), and in 3 patients associated with intraprocedural hemorrhage (dose limited to 20 mg in all 3 cases, 2 of which had symptomatic hemorrhagic transformation). In 1 case, the infusion was stopped due to an intraprocedural complication (dose limited to 15 mg alteplase). With the OAD method, alteplase delivery was curtailed in 1 patient who became hemodynamically unstable (dose limited to 51 mg alteplase) and in 4 patients in whom extravasation was noted during alteplase delivery (dose range, 2–50 mg; 1 had symptomatic hemorrhagic transformation). In all other patients, either the maximum dose limit was met or there was significant reperfusion and the operator decided to stop infusion. Mean alteplase dose by using the RMP method was 43 mg (σ = 29) and for the OAD method, 48 mg (σ = 27).
Four patients who had mRS scores of ≥3 before presentation were excluded from the mRS and 90-day mortality calculations. This exclusion included 1 patient with severe multiple sclerosis treated with the OAD method, with TICI 3 reperfusion and infarct volume of 7.6 mL; 1 patient who had significant cognitive impairment who was treated with the OAD method with TICI 3 reperfusion and infarct volume of 10.9 mL; 1 patient with a cardiac ejection fraction <10% treated with the RMP method with TICI 0 reperfusion and infarct volume of 247 mL; and 1 patient with metastatic lung cancer treated with the OAD method, with TICI 2 reperfusion and infarct volume of 0.85 mL. The latter 2 patients had life expectancies of <3 months before treatment.
Discussion
The current study demonstrates that the technique used by the operator when administering IA thrombolytic therapy influences reperfusion rates. Data collected here indicate that positioning the microcatheter to achieve a more uniform distribution of alteplase throughout the clot leads to higher reperfusion rates (Tables 2 and 3) compared with methods that randomly imbed the microcatheter within the thrombus. Because alteplase is known to bind to the surface of the clot,1 maximizing the surface exposed to this thrombolytic agent may be critical. Meticulous positioning of the microcatheter based on contrast injections demonstrating greater coverage of the thrombus, as described in this work, should result in a more favorable distribution of the thrombolytic agent and higher reperfusion rates. The microcatheters used to optimize alteplase distribution around the clot were low-profile (ie, ≤2F) and included those with floppy distal ends (Elite 1.8; Boston Scientific, Washington, DC). As a result, adjustments of the microcatheter in the complicated anatomy of some patients in this study did not pose a significant barrier to fine microcatheter repositioning. In addition, microcatheter injections were always very gentle to avoid inadvertent rupture of small perforator branches adjacent to the thrombus during injection of the alteplase. No more than 0.1–0.2 mL is required for adequate evaluation of the microcatheter position within the clot. Careful attention to alteplase infusion within the thrombus is underscored by a study suggesting that microcatheter injection may result in higher rates of hemorrhage,9 which were not found in this study.
Nominal logistic regression analysis for ≥50% reperfusion of the involved vascular territorya
Stratification of methods of IA delivery of thrombolytics by reperfusion outcome indicates a significant (P < .0001, Pearson correlation) difference between reperfusion rates using the OAD method versus RMP methoda
A microwire maceration technique was sometimes used in conjunction with RMP. The influence of wire maceration of thrombus is not clear. The microwire was removed from the thrombus before alteplase infusion; therefore, microchannels thought to have been created by microwire maceration of the clot may not necessarily remain open during the infusion. Certainly overstimulation of the vessel from microwire manipulation or microcatheter maneuvering may theoretically result in mechanical vasospasm, decreasing the ability of alteplase to access the thrombus. Various mechanical clot disruption techniques (microwire, microcatheter, balloon angioplasty, J-shaped microwire, pigtail microwire, snares, or simultaneous alteplase infusion through a large-profile microcatheter while macerating with a microwire positioned inside the microcatheter) followed by urokinase or reteplase infusion have substantially improved recanalization rates.10⇓⇓–13 Recanalization rates from 75% to 95.7%10⇓⇓–13 have been achieved by using mechanical clot disruption techniques without substantial impact on symptomatic hemorrhage rates. More sophisticated techniques or the use of thrombolytic agents with better clot penetration rates might account for different recanalization rates relative to simple microwire manipulation in the current study.
Large hemorrhage or symptomatic hemorrhagic transformation rates did not differ significantly between the methods used in this study (Table 4) or previous reports.3,4,10⇓⇓–13 The disruption of the blood-brain barrier due to free radical activated inflammatory cytokines from ischemic or reperfusion injury might be the underlying cause of hemorrhage, regardless of methodology.14 In addition, each method may have its own independent risk factor. Repeated microcatheter contrast injection may cause the extravasation of contrast to the surrounding tissue and thus result in toxic damage with resultant hemorrhage.15 As a result, operators repeating microcatheter injections should take care to reduce injection pressure and volume. Microwire maceration of the clot also carries the risk of penetration into perforator branches with resultant hemorrhage.12,16 Angiographic control allows early detection of hemorrhage in the form of intraprocedural contrast extravasation from a perforating artery near the site of drug delivery and serves as a reason to stop alteplase delivery. This was usually not associated with clinical deterioration or large hematoma when detected early but often was associated with persistent occlusion.
Stratification of outcomes by methods of IA delivery of thrombolytics
Additional factors that were associated with higher reperfusion rates included SAF, proximal location of the offending thrombus, time to treatment, and presenting NIHSS score. SAF has been shown to be associated with higher recanalization rates regardless of the method used in this study. It is hypothesized that SAF allows thrombolytic agents to surround the thrombus, thus improving distribution of alteplase. It was clear that most thrombi encountered are likely to respond to alteplase by using careful microcatheter positioning as described in this study. Distal location and internal carotid artery terminus locations of thrombus have been found to result in lower reperfusion rates.17 This outcome was also the case in this study. In 6 patients who underwent IATT for a carotid terminus occlusion by using the RMP method, none achieved TICI 2 or better reperfusion, whereas IATT for a carotid terminus occlusion by using the OAD method resulted in TICI 2 or better reperfusion in 75% of cases. Distal occlusions were shown to be associated with lower recanalization rates based on logistic regression analysis. Time to treatment <270 minutes was associated with higher (TICI 2 or TICI 3) reperfusion rates, regardless of the alteplase delivery method; however, improvement in reperfusion rate was more evident in patients undergoing IATT by using the RMP method (19.2% versus 53.3%) than in patients undergoing IATT by using the OAD method (75% versus 87.5%). This finding is consistent with the fact that a thrombus becomes more resistant to thrombolytic treatment with time.
Table 5 compares recanalization and clinical outcomes between this study and trials reported in the literature. Assessment of reperfusion in this study differs from recanalization assessment in other studies. Accurate comparison of revascularization is therefore not possible as has been explained previously by Tomsick.18 A possible exception may be the assessment for any recanalization and complete recanalization. The current study differed from the Prolyse in Acute Cerebral Thromboembolism trial because the current study included carotid terminus occlusions, distal branch occlusions, and basilar artery occlusions, whereas the Prolyse in Acute Cerebral Thromboembolism study only included M1 segment and M2 division occlusions and used a different thrombolytic agent. The Interventional Management of Stroke trial,19 the Multi Mechanical Embolus Removal in Cerebral Ischemia trial,20 and the Penumbra Pivotal Stroke trial21 included patients with higher presenting NIHSS scores and differed in their delivery method of alteplase. Finally, the current study included 16 patients in whom treatment was started in less than 3 hours from symptom onset. Presenting NIHSS score, occlusion site, and time to treatment were shown to affect reperfusion rates (Table 2). Reperfusion rates by using the OAD method appear significantly better than those reported in the literature. It is emphasized that the upper limit for the IA alteplase dose was 100 mg, which is significantly higher than the upper limit of the IA dose for the Interventional Management of Stroke trial. Higher doses of alteplase delivery need to be used with caution. Accounting for cumulative reperfusion rates for M1 and carotid terminus occlusion higher upper limit doses has been shown to improve reperfusion rates22 and increase hemorrhage rates. Furthermore, at 1 mg per minute, the alteplase delivery time is longer; however, the typical microcatheter placement time within the thrombus is more rapid than placement of a thrombectomy device. Comparison of secondary outcome measures reported in the current study with those in other trials with (Table 5) should be approached with caution due to differences of inclusion criteria. Table 5 suggests that these clinical outcomes for the Prolyse in Acute Cerebral Thromboembolism, Interventional Management of Stroke, Multi Mechanical Embolus Removal in Cerebral Ischemia, and the Penumbra Pivotal Stroke Trial are similar to outcomes reported for IATT in the current study by using the RMP method, whereas clinical outcomes by using the OAD method compared more favorably.
Comparison of outcomes from clinical trials with the current study
The current study has many limitations. A retrospective analysis introduces bias. The angiographic assessment of reperfusion and the extent of pial collateral flow was performed retrospectively at a separate sitting with the reviewer blinded to the patient information, to partially circumvent this bias. Lack of statistically significant differences does not exclude type 2 error, which would likely require a larger patient cohort. Because experience with early assessment in acute ischemic stroke has increased with time, fewer patients with a small penumbra zone will be treated because it has become clear that those patients would not benefit from treatment.7,23 As a result, the patient population presented here does not represent current patient selection criteria for treatment. This would significantly alter conclusions derived from the study with regard to reperfusion rates but may influence clinical outcomes. Finally, each method used in this study was performed by a different operator. It is likely that despite the difference in operators, different methodologies accounted for the different outcomes.
Conclusions
A method that intends to evenly distribute alteplase around a thrombus resulted in greater reperfusion rates and better clinical outcomes compared with methods without this intention. Other factors positively influencing reperfusion included the presence of slow antegrade flow distal to the clot, earlier time to treatment, lower presenting NIHSS score, and proximal occlusion site.
Footnotes
Disclosures: Andrew Slivka—UNRELATED: National Institutes of Health,* AGA Medical Corporation,* Lundbeck,* Comments: National Institutes of Health (SPS3, IRIS, MR RESCUE). *Money paid to the institution.
References
- Received July 23, 2011.
- Accepted after revision October 18, 2011.
- © 2012 by American Journal of Neuroradiology