Abstract
SUMMARY: Unexplained SDH in infants and children is an accepted marker for AHT. It has been proposed that IVT may be the initiating event leading to the development of acute SDH, mimicking the appearance of traumatic SDH. Our study aims to investigate if nontraumatic IVT causes SDH in the pediatric population. We retrospectively identified 36 patients with IVT and reviewed neuroimaging studies for the concurrent presence of SDH. In our 36 patients with IVT, no associated SDH was observed. Even with extensive IVT, no SDH was present. Three false-positive diagnoses of IVT were identified in the setting of mastoiditis and traumatic SDH, demonstrating pitfalls in imaging. In conclusion, our findings do not support the previous AHT literature stating that IVT is associated with, or leads to, SDH in neonates, infants, or children.
ABBREVIATIONS:
- AHT
- abusive head trauma
- CECT
- contrast-enhanced CT
- CI
- confidence interval
- CVT
- cortical vein thrombosis
- FOV
- field of view
- GRE
- gradient recalled echo
- ICV
- internal cerebral veins
- IVT
- intracranial venous thrombosis
- MIP
- maximum intensity projection
- SDH
- subdural hematoma/hemorrhage
- SPGR
- spoiled gradient-recalled
- TOF
- time-of-flight
IVT, which is defined as CVT and/or dural venous sinus thrombosis, is relatively rare in children and young adults. It is often associated with underlying disease states that predispose a patient to a thrombotic event. SDH is also rare in the neonate, infant, and young child, and is usually associated with trauma, infection, thrombogenic states, and less often with other causes.1 IVT occurs with an estimated prevalence of 0.67/100,000 among infants in the first year of life.2 Neonates account for a significant number of pediatric cases. Risk factors for neonatal IVT include polycythemia, other prothrombotic states, and perinatal complications such as head trauma, asphyxia, sepsis, and meningitis. Spontaneous IVT has been described in infants and children, and is frequently due to an underlying condition such as infection, shock, dehydration, hypercoagulable states, iron deficiency anemia, and tumors.3 However, in a review of the literature, very few cases of IVT were associated with SDH.
AHT in neonates, infants, and young children is a complex diagnosis, requiring the interaction of pediatrics, radiology, ophthalmology, and the child protection team. Commonly, young infants present for medical care with unexplained neurologic symptoms, and the diagnosis of a SDH is made based upon imaging. The diagnosis of AHT is then subsequently established through a process that ties medical opinions with history, assessment of other injuries, and investigative results. Patients with AHT may develop IVT as a consequence of intracranial injury. Most such patients also often have other indicators of abusive injury such as fractures, bruises, and retinal hemorrhages. Only recently has IVT been proposed as the initiating event for extra-axial hemorrhage, such as SDH and subarachnoid hemorrhage, in the pediatric population. The theory suggests that spontaneously developing IVT leads to SDH, which is similar to the presentation of a child with a traumatic SDH. Therefore, in the absence of a history of a traumatic mechanism, when SDH and IVT are seen together, this theory suggests that IVT is the cause of SDH rather than the effect. The mechanism proposed is that IVT causes venous backpressure, leading to extravasation of blood into the subdural spaces.4⇓⇓–7 Head trauma, however, is a well-recognized factor in the development of IVT, with direct damage to the venous sinuses. Complications of severe head trauma—such as sutural diastasis and/or skull fracture with mechanical compression or disruption of the venous sinus, shock, cerebral edema, poor cerebral perfusion, and coagulopathies—are also associated with the development IVT.8
Our hypothesis is that if extra-axial hemorrhage is the result of IVT, then children with IVT from nontraumatic causes would be at risk to develop subdural collections. Our review of the pediatric literature revealed no previous study that systematically addressed the association of nontraumatic pediatric IVT with convexity SDH in children beyond the neonatal period. DeVeber analyzed 160 cases of IVT from the Canadian Pediatric Ischemic Stroke registry. In this study, 9/160 children with IVT were described as having extra-axial collections, with no further description. Our personal communication with the author indicates that most of these collections occurred in the neonatal period and were found in the parieotoccipital, paratentorial, and posterior fossa regions—typical locations for birth-related SDH.2 Sébire et al prospectively analyzed 42 cases of non-neonatal IVT, where most patients had mastoiditis and others were dehydrated, infected, or both.9 Heller et al evaluated 149 children (neonate to 18 years old) with IVT, outlining the multifactorial nature of IVT in children. Of this series, 70% revealed an underlying risk factor for the development of IVT, none of whom had accompanying SDH.10
Older children and adults with IVT have risk factors such as head trauma as well as hypercoagulable states, which may be genetic (such as Protein C, Protein S, and antithrombin III deficiencies) or due to diseases such as systemic lupus erythematosis, nephrotic syndrome, leukemia, or other malignancies.6 Spontaneous IVT in adults is also a rare event. The association of IVT in adults with SDH appears to be more complex. A case report of SDH developing as the result of sagittal sinus thrombosis occurred during anticoagulation therapy for the thrombosis.6 Singh et al described a case of a 36-year-old woman presenting with SDH as a result of chronic cerebral vaso-occlusive disease.7 Oral contraceptives are a known risk factor for IVT, as well as tumors, drugs, and pregnancy. Intracranial hemorrhage is described as a complication in adults with IVT but is often intraparenchymal in location and considered to be the result of hemorrhagic transformation of venous infarction.11
The purpose of this study is to review a series of children and young adults from a tertiary children's hospital with intracranial venous thromboses, where neuroimaging was available for review, to determine whether IVT is associated with SDH.
Materials and Methods
With institutional review board approval, the imaging archive and electronic medical record at a tertiary pediatric medical center was searched for the key words “CVT” and “dural venous sinus thrombosis.” For the purposes of this study, IVT was defined as CVT and/or dural venous sinus thrombosis. Using this method, 42 patients were identified from January 2003 to December 2010. An attending pediatric neuroradiologist and a neuroradiology fellow retrospectively reviewed all cerebral cross-sectional imaging of these patients for IVT. Confirmation of suspected IVT was made by consensus agreement between the 2 neuroradiologists based upon the presence of venous intraluminal filling defects observed after assessing either contrast-enhanced CTV or enhanced MRV. Next, an attending pediatrician reviewed the clinical histories of these patients. Of 42 patients identified using this method, 6 patients were excluded from our study because, on subsequent imaging evaluation, they did not have IVT.
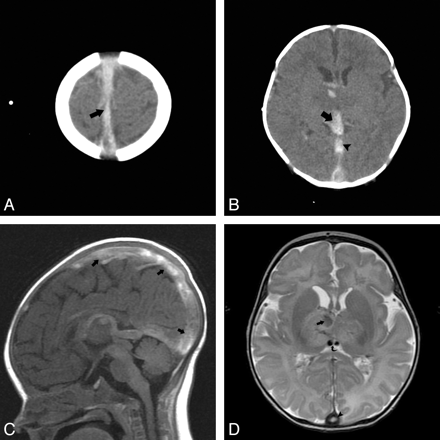
A total of 36 patients with IVT were included for analysis. There were 16 males (44%) and 20 females (56%). The age range was from 1 day of life to 19 years old (On-line Table ). IVT was diagnosed by cross-sectional imaging: CT only, 2/36 (5.6%); MR imaging only, 14/36 (38.9%); and both CT and MR imaging, 20/36 (55.6%). In the 2 patients who had IVT diagnosed by CT alone, IVT diagnosis was established with CT venography and CECT, respectively. All other patients had MR imaging to confirm the diagnosis of IVT. The extent of IVT in the study population was isolated to 1 dural venous sinus in 7/36 patients. IVT involved >4 of the intracranial venous sinuses in 7/36. One patient in our study group had extensive IVT that resulted in venous infarction (Fig 1).
Fourteen-day-old male with a neonatal seizure due to methylenetetrahydrofolate deficiency. A, Axial noncontrast CT through the cerebral convexities shows hyperattenuated clot in the superior sagittal sinus (arrow). B, Note the thrombosed ICVs converging with the thrombosed vein of Galen (arrow). The straight sinus is also thrombosed (arrowhead). There is loss of gray–white differentiation and intraparenchymal hemorrhage in the right basal ganglia. C, Sagittal T1 confirms the extensive IVT (arrows). D, Axial T2 image demonstrates venous infarction in the right deep gray nuclei with hemorrhagic transformation (arrow). Hypointense clot is seen in the ICVs (curved arrow). Note the clot in the posterior inferior sagittal sinus (arrowhead). No subdural hemorrhage is observed.
Noncontrast brain CT was performed in our institution on an Aquilion 64-section CT scanner (Toshiba Medical Systems, Tokyo, Japan) at 5-mm section width. Brain MR imaging was acquired on either a 1.5 or 3T Hdx MR imaging platform (GE Healthcare, Milwaukee, Wisconsin). The MR brain imaging protocol included axial DWI, T2WI, T2 FLAIR, T1 FLAIR, DTI, sagittal T1WI FLAIR, coronal T2, and coronal GRE. Optional MR imaging brain sequences that would be acquired after IV contrast included fat-saturated axial and/or coronal T1WI.
CTV was performed following 2 mL/kg of intravenous Isovue 370 (Bracco, Princeton, New Jersey) injection with a scan delay of 50 seconds, then helical scanning at 1-mm thickness; axial reconstructions at 2 mm with coronal MIPs generated at 5-mm by 1-mm intervals; and finally, reformatted coronal/sagittal images at 2-mm thickness.
Enhanced MRV was performed following 0.2 mL/kg intravenous Multihance (Bracco) injection. Sagittal enhanced 3D SPGR imaging was accomplished with TE in phase, TI of 300 msec, flip angle of 18, and band width of 31.25. Enhanced MRV parameters included a 24.0-cm FOV, and 2-mm section thickness. Acquisition timing included frequency of 256, phase of 192, and 1 NEX. In cases of 2D TOF, MRV was performed and suspected IVT was confirmed with either CECT or MR imaging.
The cross-sectional neuroimaging studies were then examined for the presence of concurrent SDH. We found that 0/36 patients had associated SDH (95% CI, 0–9.7%). Of note, although SDH can be seen in birth-related trauma in the neonatal population, our study was designed to identify patients with IVT, none of whom had concurrent SDH. The elapsed time between initial CT brain imaging and confirmatory enhanced CT, CTV, or MR imaging/MRV was less than 72 hours.
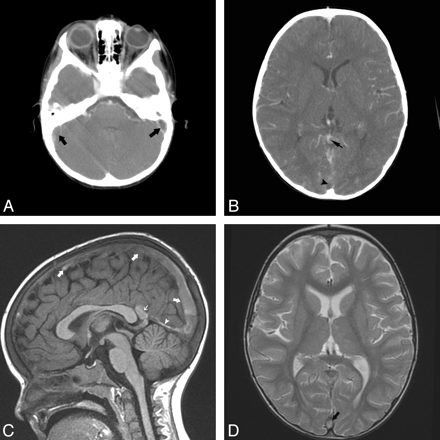
The medical condition, or risk factor for IVT, varied among our subjects (On-line Table). All 36 patients identified in our study had nontraumatic IVT. Further analysis revealed that in 13/36 subjects, infection was the etiology for thrombosis. These patients were diagnosed with sinusitis, otitis media, mastoiditis, or urinary tract infections. The next most common diagnosis associated with IVT was dehydration, which was the risk factor for thrombosis in 6/36 patients. Underlying malignancy was found in 5/36 patients, all of whom had a diagnosis of leukemia. Iron deficiency anemia was implicated as the cause of IVT in 4/36 patients. Lastly, in the older teenage population, oral contraceptives were the cause of IVT in 3/36 patients (Fig 2).
Two-year-old girl with severe iron deficiency anemia presented with extensive IVT. A and B, Axial CT angiogram images show filling defects in both sigmoid sinuses (arrows) and thrombus within the vein of Galen (small arrow) and posterior superior sagittal sinus (arrowhead). C, Sagittal TI image shows TI hyperintense clot throughout the superior sagittal sinus (arrows), ICV, vein of Galen (small arrow), and straight sinus (arrowhead). D, Representative axial T2 image shows no evidence of subdural hemorrhage. Note the hyperintense clot within the posterior superior sagittal sinus (arrow).
Discussion
It has been proposed that spontaneous IVT may precipitate SDH in infants and children, resulting in SDHs that appear similar to traumatic SDHs seen in AHT.12,13 The theory that dural venous sinus thrombosis leads to increased pressure or tension in cortical veins and thus results in subdural bleeding was not observed in any patient in this retrospective study.4⇓⇓–7,14 Our careful review of case reports that suggest IVT as an initiating event leading to SDH reveals that they barely satisfy the criteria for level III quality data, as established by the US Preventive Services Task Force. In fact, even in 1937, Bailey and Hass elegantly described 3 pediatric patients with IVT, 2 of whom had SDH at autopsy. In the authors' words, they could “establish no absolute interdependence of the original thrombus in the superior longitudinal sinus and the intracranial hemorrhage.”4 Bucy and Lesemann's report, in 1978, of a patient with idiopathic recurrent thrombophlebitis and SDH did not comment upon the use of therapeutic anticoagulation as a potential risk factor for SDH and presumed a causal relationship between IVT and SDH.5 More recently, in 1982, Matsuda et al reported the occurrence of IVT and SDH in a 33-year-old woman.6 Interestingly, the patient had received urokinase before the diagnosis of SDH; the patient's coagulation status at the time the SDH was diagnosed was not reported. Takamura et al reported on a 35-year-old man with multiple systemic hemangiomas, cerebral venous thrombosis, consumptive coagulopathy, and chronic left SDH.14 The authors postulated that collateral venous pathways caused hemodynamic stress and rupture of bridging veins, leading to SDH. In addition, the authors reported multiple areas of dural-based enhancement on MR imaging, leading to speculation that if the dura were involved with cavernous hemangiomata, the patient would reasonably be predisposed to develop SDH. In 2005, Singh et al reported on a 39-year-old man with IVT and bifrontal hygromas.7 No mention was made of antecedent trauma or whether the brain MR imaging evaluation included GRE imaging to assess for the presence of blood products.
None of the previous reports have substantiated the claim that increased intracranial pressure, either acute or chronic, resulting from IVT may play a role in causing SDH.15 Neither the previous mentioned case reports nor the autopsy series of Bailey and Hass described findings to support pre-existing elevated intracranial pressure.4⇓⇓–7,14 Other clinical and imaging observations observed in conjunction with IVT include brain herniation syndromes, cerebral ischemia, or strokes that one might observe with acute states of intracranial hypertension, secondary to acute IVT, were also not described.4⇓⇓–7,14,15 Therefore, in concordance with the literature, our study also does not support the correlation of IVT with SDH. It remains an untenable position that, in a child who presents with a traumatic SDH and then subsequently develops IVT, IVT is the cause and not the result of the injury. AHT can be a difficult diagnosis to make in an in infant or young child. There is rarely a history of trauma that correlates with the severity of the head injury, and other indicators of abusive injury are often present.
In our retrospective observational study of pediatric patients with isolated and extensive IVT, an association of IVT with SDH was not identified. We postulated that if venous backpressure in the setting of IVT had a causal relationship to SDH, we would expect to see SDH, particularly in our patients with extensive IVT. This was not the case in our study. In addition, 3 of our cases, which were excluded due to an absence of IVT, demonstrated a valuable point in the imaging of suspected IVT. In 2 cases excluded from our series, on noncontrast CT brain imaging, SDH—as well as epidural empyema—mimicked dural venous sinus thrombosis when, in fact, the venous sinus was compressed by the blood or empyema. In our excluded cases, the diagnosis of IVT was initially erroneously made on noncontrast CT. However, in each case where MRV was performed, IVT was not confirmed, and the diagnosis of SDH and/or epidural empyema was ultimately correctly established. These 3 cases reinforce an important point: When SDH, due to head trauma (abusive or accidental), is suspected, MR imaging and possibly enhanced MRV often have a critical role in accurately defining the character of hemorrhage and its location, assessing the adjacent brain parenchyma, and confirming venous patency.
The limitations of our study include the small subject group and retrospective design. Prospective studies to evaluate for the presence of IVT in the setting of documented AHT would be valuable to support our findings. In conclusion, in our study of pediatric patients, nontraumatic isolated and extensive IVT was not associated with subdural hemorrhage. Therefore, we conclude that IVT does not appear to be the initiating event for SDH. This study emphasizes the importance of having suspected head trauma cases read by a radiologist familiar with pediatric neuroimaging and the CT/MR imaging features of AHT. Collaboration with a clinician experienced in pediatric medicine and child abuse issues is also important for clinical corroboration.
Footnotes
-
Disclosures: Gary Hedlund—UNRELATED: Royalties: Amirsys, Comments: Royalties paid as an author. Lori Frasier—UNRELATED: Expert Testimony: Various jurisdictions, Comments: As a child abuse pediatrician, legal testimony is part of my work. Occasionally I am asked to consult for prosecution, defense, plaintiff in criminal or civil proceedings for which I receive consulting fees; Grants/Grants Pending: Various jurisdictions, Comments: As a child abuse pediatrician, legal testimony is part of my work. Occasionally I am asked to consult for prosecution, defense, plaintiff in criminal or civil proceedings for which I receive consulting fees; Payment for Lectures (including service on speakers bureaus): Various academic and professional meetings, Comments: I am occasionally paid a modest honorarium for lectures relating to child maltreatment and AHT; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Various professional and academic meetings, Comments: Travel and expenses are often paid for invited presentations.
References
- Received October 9, 2011.
- Accepted after revision November 4, 2011.
- © 2012 by American Journal of Neuroradiology