Abstract
BACKGROUND AND PURPOSE: Some patients with SIH have fast CSF leaks requiring dynamic CTM for localization; however, patients generally undergo conventional CTM before a dynamic study. Our aim was to determine whether findings on head MR imaging, spine MR imaging, or opening pressure measurements can predict fast spinal CSF leaks.
MATERIALS AND METHODS: A retrospective review was performed on 151 consecutive patients referred for CTM to evaluate for spinal CSF leak. Head MR imaging was evaluated for diffuse dural enhancement and “brain sag,” and spine MR imaging for presence of an extradural fluid collection. The opening pressure was recorded. The CTM was scored as no leak, slow leak localized on conventional CTM, or fast leak that required dynamic CTM.
RESULTS: Fast CSF leaks were identified in 32 (21%), slow leaks in 36 (24%), and no leak in 83 (55%) of 151 patients on initial CTM. There was significant association between spinal extra-arachnoid fluid on MR imaging and the presence of a fast leak (sensitivity 85%, specificity 79%, P < .0001). There was not significant association between fast leak and findings on head MR imaging (P = .27) or opening pressure (P = .30).
CONCLUSIONS: If all patients with spinal extra-arachnoid CSF on MR imaging had been sent directly to dynamic CTM, repeat myelography would have been avoided in most patients with fast leaks (23 of 27; 85%). However, a minority of patients with slow or no leaks would have been converted from conventional to dynamic CTM (16 of 77; 21%). Spinal MR imaging is helpful in premyelographic evaluation of SIH.
ABBREVIATIONS:
- AUC
- area under the curve
- CTM
- CT myelography
- SIH
- spontaneous intracranial hypotension
While some patients with SIH recover without intervention or display a self-limited course, many do require an invasive therapeutic intervention.1 In those patients who do not respond to multiple large-volume epidural blood patches, targeted epidural blood patches, targeted fibrin glue injections, or surgical repair may be necessary. In these patients, localization of the actual site or sites of CSF leak is critical for guiding therapy. In many patients, the site of leak can be localized using conventional CTM. When there are multiple leaks or large dural tears, the time delay during transfer between the myelographic portion of the examination performed with fluoroscopy and the CT portion of the examination allows the extra-arachnoid contrast to diffuse over multiple spinal levels, thus limiting the ability to localize the leaks to within 2 spinal segments. We define these as high-flow or fast leaks, which require dynamic CTM to localize.2
When performing dynamic CTM, a spinal needle is either placed under fluoroscopic guidance, and then the patient is transferred from the fluoroscopy suite to a CT scanner, or the spinal needle is placed under CT guidance. The myelographic contrast is then injected with the patient in the CT scanner. This allows for immediate CT acquisition following contrast injection and localization of fast CSF leaks.2,3 Because multiple CT acquisitions are performed, dynamic CTM is associated with a higher radiation dose and is performed without the benefit of a tilting table. Therefore, in the past, we have advocated conventional CTM before considering a dynamic study.2 The aims of this study were to determine how frequently dynamic CTM must be performed following the initial conventional CT myelogram in order to localize fast CSF leaks, and to determine whether findings on head MR imaging, spine MR imaging, or opening pressure measurements can predict fast spinal CSF leaks in a large case series.
Materials and Methods
Patient Population
Institutional review board approval with waived consent was obtained for this Health Insurance Portability and Accountability Act–compliant retrospective research study. A search of the radiology information system identified 151 consecutive patients with clinical suspicion of SIH who were referred for CTM to evaluate for spinal CSF leak between February 2002 and August 2010. A retrospective review of the imaging and electronic medical record was performed on these 151 patients.
Imaging Analysis
If a patient had more than 1 head MR imaging or more than 1 spine MR imaging, the most recent head and spine MR imaging examinations before the first CTM were used. For each patient, the time interval between the relevant, available MR imaging examinations and the first CTM were recorded. Each head MR imaging was scored on a 3-point scale: “classic” brain MR imaging appearance of SIH with diffuse dural enhancement and “brain sag”; “partial” findings of either diffuse dural enhancement or evidence of “brain sag”; or “normal” without evidence of dural enhancement or “brain sag.” The levels examined for each spine MR imaging were recorded. Each spine MR imaging was scored on a binary scale for presence or absence of an extra-arachnoid collection. The opening pressure, when measured, was recorded. If the patient had more than 1 conventional CTM, the first CTM was used. The CTM was scored on a 3-point scale: no leak, a slow leak that could be localized to within 2 vertebral segments on conventional CTM, or a fast leak that required dynamic CTM for localization. If delayed images were obtained during the CTM, the time interval between immediate and delayed imaging was recorded, and the presence of leaks detected only on delayed images were noted. Six patients had only a dynamic CTM. In these cases, a single board-certified neuroradiologist (P.H.L.) reviewed the studies. If there was rapid diffusion of contrast into the extra-arachnoid space over more than 2 spinal segments during the acquisition of the dynamic series, the leak was scored as fast.
Statistical Analysis
For the primary analysis, leak intensity was dichotomized into either a fast leak or a slow or nondetectable leak. This reflected the binary clinical decision of performing either a dynamic CTM (optimal when a fast leak is present) or conventional CTM (optimal when a slow leak or no leak is present). Diagnostic accuracy of MR imaging readings in the head and spine were assessed by sensitivity, specificity, and the concordance index (“c-statistic”/AUC). For sensitivity and specificity, 95%-score confidence intervals were computed, and for the concordance index, the large-sample 95% confidence interval was calculated. Due to the fact the study was retrospective, complete data were not obtainable on all patients. When considering each diagnostic test individually, all available data were used. For the final analysis, however, the subset of patients with both head and spine MR imaging was utilized. All analyses were conducted using the SAS System version 9.2 (SAS, Cary, North Carolina).
Results
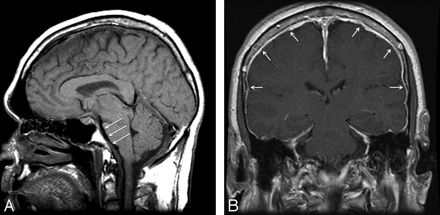
Fast CSF leaks were identified in 32 (21%), slow leaks in 36 (24%), and no leak in 83 (55%) of 151 patients during initial CTM (Table); 145 (96%) began with a conventional CTM, while 6 (4%) went directly to dynamic CTM; 27 (18%) had both a conventional and a dynamic CTM. Head MR imaging was performed before CTM in 136 (90%) of 151patients. The median time between head MR imaging and CTM was 67 days (0–1434, mean 67). Classic findings of SIH including dural enhancement and “brain sag” were present in 68 (50%; Fig 1), findings of either dural enhancement or “brain sag” were found in 30 (22%), and no dural enhancement or “brain sag” in 38 (28%) of 136 patients. There was not significant association between fast leak and findings on head MR imaging (χ2 = 2.6, df = 2, P = .27). Consistent with this observation, the overall discrimination as measured by the concordance index was poor (c = 0.59, 95% CI, 0.49 to 0.69).
Association of CSF leak rates by pre-myelographic screening exam
Classic findings of SIH on MR imaging of the brain with “brain sag” demonstrated on midline sagittal T1 image (A), including descent of the cerebellar tonsils below the foramen magnum, flattening of the ventral pons (white arrows), and inferior displacement of the optic chiasm (open arrow). Postgadolinium coronal T1 image (B) demonstrates diffuse dural enhancement (white arrows.)
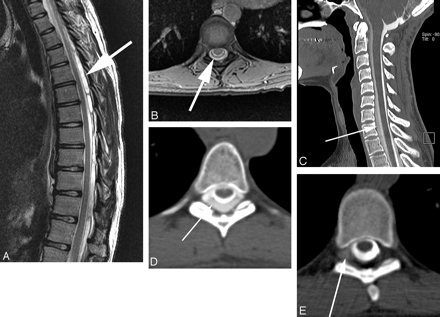
Spine MR imaging was available in 104 (69%) of 151 patients; 76 (73%) patients had imaging of the entire spine, 13 (13%) had only 2 segments, and 15 (15%) had only 1 segment examined. The median time between spine MR imaging and CTM was 7 days (range 0–393, mean 45). Extra-arachnoid spinal fluid was present in 39 (38%) (Fig 2A, B) and absent in 65 (63%) of 104 patients with spine MR imaging. There was a significant correlation between the presence of spinal extra-arachnoid fluid and the presence of a fast leak (χ2 = 35.4, df = 1, P < .0001; Figs 2 and 3). The sensitivity and specificity of extra-arachnoid spinal fluid for the detection of fast leaks were 85% (95% CI: 68% to 94%) and 79% (95% CI: 69% to 87%), respectively. Discrimination of extra-arachnoid spinal fluid was high (c = 0.82, 95% CI: 0.74 to 0.90).
Fast CSF leak in a patient with extra-arachnoid fluid on spinal MR imaging, localized on dynamic CTM. Large extra-arachnoid fluid collection is seen on sagittal T2 images of the thoracic spine, predominantly posterior to the thecal sac (A), which involved the entire cervical and thoracic spine. Axial gradient images through the spine confirm extra-arachnoid fluid (B). Conventional CTM shows a collection of contrast outside of the thecal sac (C, D), which extends from C3 to L4 and the leak cannot be localized. Dynamic CTM shows extraarachnoid extravasation of contrast on the right at T6-T7 (E) and also at right T7-T8 (not shown), consistent with 2 sites of CSF leak.
Fast CSF leak with spinal extra-arachnoid fluid collection requiring dynamic CTM for leak localization. Fluid collection ventral to the thecal sac is best seen on axial T2-weighted images (A). This collection is smaller than the collection seen in Fig 2 and involves only the lower cervical and upper thoracic spine. Conventional CTM (B) shows a ventral contrast collection from C3 through T9, but the site of leak cannot be specified. Dynamic CTM (C) shows extra-arachnoid contrast accumulating ventral to thecal sac at C6-C7, consistent with a ventral leak at this level.
Both spine MR imaging and head MR imaging were available in 98 (65%) of 151 patients. Using a combination of a “classic” head MRI with diffuse dural enhancement, and “brain sag” and extra-arachnoid fluid on spine MR imaging to predict a fast leak resulted in a much lower sensitivity without significant increase in specificity (sensitivity 46%, 95% CI: 29% to 65%; specificity 89%, 95% CI: 80% to 94%) compared with using extra-arachnoid spinal fluid alone. Overall discrimination of this combination of characteristics was 0.16 percentage points lower than that of the spine results alone (P = .001).
Opening pressure measurements were performed in 76 (50%) of 151 patients. The mean opening pressure measurement was 108 mm of water (0–250, median 108). Very low opening pressure measurements (≤50 mm water) were found in 16 patients (21%), low (51–100 mm water) in 21 (28%), normal (101–180 mm water) in 33 (43%), and high (>180 mm water) in 6 (8%). There was not a significant correlation between fast leak and opening pressure (χ2 = 3.6, df = 3, P = .30).
Of the145 patients with conventional CTM, 67 patients with slow or no leak on initial CTM images had delayed images, with an average delay of 3 hours. A leak was detected only on the delayed images in 1 (1.5%) of 67 patients. Delayed images were not obtained on any patient with fast leak.
Discussion
Over the past 15 years, SIH has been diagnosed in an increasing number of patients, and a broader clinical and imaging spectrum of the disorder has been recognized.1,4⇓⇓–7 The combination of postural headaches and typical changes on head MR imaging—which include diffuse pachymeningeal enhancement, subdural fluid collections, and “sagging” or descent of the brain associated with descent of the cerebellar tonsils, partial effacement of basal cisterns, and inferior displacement of the optic chiasm—suggests the diagnosis of SIH.4 In our experience, the greatest diagnostic challenge is no longer the recognition of SIH but the confirmation and localization of the site of CSF leak.
Most, if not all, cases of SIH result from spontaneous CSF leaks, and most of these occur in the spine.8 The disorder is often self-limited and responds to conservative therapy and/or large-volume nontargeted lumbar blood patches. However, in those patients who fail conservative or nontargeted therapy, localization of the precise site of the leak has critical therapeutic implications. The rate of CSF leak can vary tremendously and is difficult to predict. A subset of patients have fast leaks that are not localized by conventional CTM, and both dynamic CTM2 and digital subtraction myelography9 have been reported as useful in localizing rapid leaks. Both of these techniques require more time and technical expertise, and use a larger radiation dose than conventional CTM. Thus, conventional CTM has been advocated as the initial examination for localization of CSF leaks.2
In this study, we demonstrate that fast CSF leaks occur in a significant portion of patients referred for CTM in a large tertiary medical center. We show that the presence of extra-arachnoid fluid on premyelographic spinal MR imaging can be used to predict the presence of a fast CSF leak. In our patient population, repeat CTM could have been avoided in 23 (85%) of 27 patients with fast leaks if patients with spinal extra-arachnoid fluid would have been sent directly to dynamic CTM. This is significant, as the radiation associated with the first CTM could be avoided. In addition, the risk and discomfort associated with repeat injection of intrathecal contrast over a short time interval could also be avoided. The patient's itinerary would not be delayed by the time interval required to allow the first dose of intrathecal contrast to be resorbed from the subarachnoid space. Although 16 (21%) of 77 patients with slow or no leaks would have been converted from conventional to dynamic CTM with an increase in radiation dose, the benefits associated with avoiding repeat studies outweigh the risks associated with converting these relatively few patients to dynamic CTM.
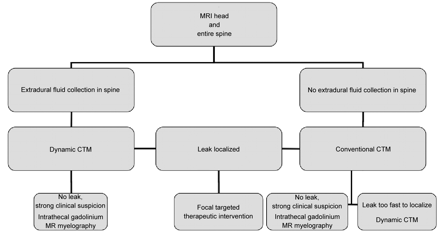
We propose a new imaging algorithm for the localization of spinal CSF leaks in those patients who fail conservative management and nontargeted therapy, incorporating MR imaging of not only the head but also the entire spine before CTM (Fig 4). Patients should proceed to conventional CTM if there is no extra-arachnoid spinal CSF collection, or proceed directly to dynamic CTM if an extra-arachnoid spinal fluid collection is present. Intrathecal gadolinium MR myelography continues to be a useful technique in the highly selected group of patients with debilitating symptoms of SIH and a negative conventional CTM.10 Although we have historically performed delayed imaging at 3–4 hours after intrathecal injection of contrast when initial imaging fails to show a leak, this proposed algorithm also removes delayed CTM images, as delayed imaging adds radiation dose and does not add significant value, based on the results of our study.
Imaging algorithm in persistent spontaneous intracranial hypotension unresponsive to conservative management and nontargeted therapy.
Previous authors have described the findings of SIH in the spine, which include the presence of extra-arachnoid fluid, pachymeningeal enhancement, and enlargement of the epidural venous plexus.1,4,11,12 However, to our knowledge, the ability of spinal MR imaging to predict fast CSF leaks has not been previously described.
While head MR imaging is helpful in confirming the clinical diagnosis of SIH and in excluding other causes of headache, the absence of pachymeningeal gadolinium enhancement on head MR imaging, despite symptomatic CSF leak, has been previously reported.13 In addition, the variability of CSF opening pressure has also been observed.4 Our study supports these observations and demonstrates the lack of association between findings on head MR imaging or opening pressure and the rate of CSF leak.
Our study limitations include those inherent to a retrospective analysis and single-center experience. Both head and spinal MR imaging were not performed in all patients, and the entire spine was not imaged in all cases. In some cases, there was a significant time delay between the MR imaging examinations and CTM. This lack of uniformity in pre-CTM imaging may introduce a selection bias. Likewise, opening pressures were not obtained in all cases. A prospective study that includes MR imaging of the head and entire spine within a week of CTM, and which includes opening pressures on all patients, would be helpful in confirming our findings.
Conclusions
Fast CSF leaks occurred in 21% of our patients with SIH, and repeat dynamic CTM was performed in 18%. Repeat CTM would have been avoided in 23 (85%) of 27 patients with fast leaks if patients with spinal extradural CSF were sent directly to dynamic CTM. However, 16 (21%) of 77 patients with slow or no CSF leaks would have been converted from conventional to dynamic CTM, with associated increased radiation exposure. Based on these results, we propose a new algorithm for evaluation of patients with SIH, which incorporates MR imaging of the entire spine and proceeds directly to dynamic CTM rather than conventional CTM in patients with spinal extradural CSF collections noted on MR imaging.
Footnotes
-
This data has been accepted for oral presentation at the 2011 American Society of Neuroradiology Annual Meeting, June 6, 2011, Seattle, Washington.
References
- Received June 10, 2011.
- Accepted after revision July 16, 2011.
- © 2012 by American Journal of Neuroradiology