Abstract
BACKGROUND AND PURPOSE: DW-MRS is a promising tool for the noninvasive identification of the cellular response to cerebral ischemia. To date, the potential confounding effects of aging and the stage of ischemia are unknown. We, therefore, examined the cross-sectional effects of age and different stages of cerebral ischemia on the diffusion of brain metabolites.
MATERIALS AND METHODS: The ADCs of 3 major metabolites, including Cho, Cr, and NAA were measured by DW-MRS in healthy younger (n = 26, 24 ± 2.2 years of age) and older (n = 17, 63 ± 7.0 years of age) adults, as well as in patients with acute (n = 7, 57 ± 4.0 years of age) and subacute (n = 12, 62 ± 7.8 years of age) cerebral ischemia.
RESULTS: Compared with younger adults, healthy older adults presented with significantly reduced ADC values of NAA (P = .000052), Cr (P = .000018), and Cho (P = .00075). Meanwhile, the ADC values of NAA (F2,36 = 6.057, P = .006), Cr (F2,36 = 5.634, P = .008), and Cho (F2,36 = 8.167, P = .001) were significantly different among the acute cerebral ischemia group, subacute cerebral ischemia group, and healthy older controls. These metabolites decreased in the acute stage of cerebral ischemia but increased in the subacute stage, compared with age-matched controls.
CONCLUSIONS: The effect of age should be considered when analyzing diffusion of cerebral metabolites with DW-MRS. Our observations also suggest that metabolite diffusion data may be used to reveal changes in the intracellular environment, depending on the pathologic status of ischemia.
ABBREVIATIONS:
- DW
- diffusion-weighted
- FID
- free induction decay
- PRESS
- point-resolved spectroscopy sequence
Cerebral ischemia is a major cause of death and disability in industrialized countries.1 It is the third leading cause of death after heart disease and cancer2 and affects 1 million Europeans each year.3 In the United States, approximately 700 000 persons have a stroke annually, among whom 25% will die, and most of the survivors will have associated morbidity and functional limitations.4
With the development of imaging technology, DWI and MR imaging have been widely applied in the diagnosis and treatment of cerebral ischemia. DWI can detect early ischemia in cerebrovascular accidents and characterize both intracranial infections and brain tumors.5,6 Because water molecules can diffuse freely and exchange between both extra- and intracellular compartments, the water ADC values reflect a weighted result of the contributions from both of them.7 It also means that discrimination between intra- and extracellular contributions by standard DWI would be difficult.8 As a result, the standard DWI technique is not suitable for detecting the specific changes of the intracellular space of the tissues. In vivo MRS offers images of the metabolites that are mainly located in the intracellular compartment, which provides the possibility of obtaining diffusion information of the intracellular space of tissues. In particular, DW-MRS,9,10 the combined technology, is of fundamental interest. It permits evaluation of the intrinsic diffusion properties of the metabolites, NAA, Cr, and Cho, which are exclusively located in the intracellular space and exchange very slowly between the 2 compartments.11,12 Consequently, the diffusion in the intracellular space is the only factor of the detected ADCs of metabolites13 that may indicate changes in the cell.
Previous studies indicated that DW-MRS may provide insight into the tissue response to numerous disorders, including cerebral ischemia.14⇓–16 One previous report indicated that the ADCs of NAA and Cr within cerebral tissue under conditions of acute infarction were decreased compared with those in controls.14 On the other hand, a different report indicated that ADC values of NAA were increased in the human brain during the subacute phase of infarction, remaining elevated for ≤60 days following the injury.17 The physiologic mechanism underlying these discrepancies is currently unclear.
Biologic aging from adulthood into senescence is associated with cellular damage and may therefore independently influence metabolite diffusion.18⇓⇓–21 Previous in vivo examinations have demonstrated significant effects of aging on the number, geometric shape, and volume of cortical neurons. Associated alterations in diffusion space may, therefore, affect the characteristics of metabolite diffusion.22⇓–24
Therefore, the goal of this study was to determine the cross-sectional effects of both age and duration from the onset of cerebral ischemia on the ADCs of cerebral metabolites, by using PRESS-based DW-MRS sequences on a 3T MR imaging scanner. We aimed to find out the following: 1) whether aging is closely associated with the ADCs of metabolites in healthy volunteers, and 2) whether the different stages of cerebral ischemia will affect the level of diffusion of metabolites.
Materials and Methods
Subjects
Forty-three healthy volunteers and 19 patients with cerebral ischemia were recruited for this study (Table 1). Because the rate of brain shrinkage accelerates after of 50 years of age,25 healthy volunteers were separated into younger (younger than 50 years, n = 26) and older groups (older than 50 years, n = 17). Of the 19 patients (older than 50 years) with partial anterior circulation syndrome, 7 were in the acute stage (ie, between 24 and 48 hours from onset of symptoms) and 12 were in the subacute stage (ie, at least 72 hours from the onset of symptoms). In all patients, the presence of ischemia was confirmed by hyperintense regions using DWI-MRS scans read by a radiologist with 5 years' experience. All infarctions were caused by occlusions of a branch of the MCA.
Healthy young, healthy older, acute ischemia, and subacute ischemia group demographics and stroke characteristics
Exclusion criteria for the healthy controls were the following: 1) a history of headaches, 2) the presence of epilepsy, 3) any organic disease in the brain detected by MR imaging, 4) hypertension, 5) cancer, 6) diabetes mellitus, 7) any unstable medical conditions, 8) Mini-Mental State Examination scores of <29, and 9) any contraindications to MR imaging. All patients with ischemia were referred to the study by the radiologists and physicians affiliated with the hospital.
In this study, all healthy subjects and patients gave their informed consent, which was approved by the Investigational Review Board at Peking University First Hospital.
Pulse Sequence
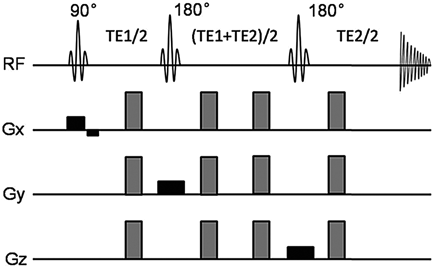
A DW-MRS sequence based on the PRESS sequence technique15 was implemented for the DW-MRS clinical studies. Two pairs of diffusion gradients were positioned symmetrically around the two 180° refocusing pulses in 3 directions (Fig 1).26 This design is helpful to achieve high b factor with lower gradients. Furthermore, it reduces the effects of eddy currents, which are induced when the gradient switches.15 Before excitation radio-frequency, a chemical shift selective water-suppression radio-frequency pulse sequence27 was completed to suppress the water signal intensity.
Schematic radio-frequency pulse and diffusion gradients for diffusion-weighted PRESS. The diffusion-weighted gradients are present around the second and third 180° pulses. The amplitudes are fixed at 80% of the maximum amplitude of the system, and the gradients of 3 directions are used together to increase the b-value.
The total measurement time for a set of DW-MRS series was 8 minutes 24 seconds. The volunteers' and patients' heads were fastened by using numerous vacuum pillows to minimize the macroscopic motion.
MR Imaging Protocol
A 3T clinical whole-body system (Signa Excite HD; GE Healthcare, Milwaukee, Wisconsin) was used to acquire MR images. Maximum gradient amplitudes were 40 mT/m, and maximum slew rates were 150 mT/m/ms. An 8-channel high-resolution head coil was used, which is able to perform both proton MR imaging and MRS.
The MR imaging acquisition protocol included the following sequences: localizer, DWI, T1 FLAIR, T2 FLAIR, and DW-MRS.
The DWI parameters were TR/TE, 4000/60 ms; FOV, 24 × 24 cm; matrix size, 128 × 128 pixels; NEX, 2; and b factors, 0 and 1000 s/mm2. The T1 FLAIR parameters were TR/TE, 2300/10 ms; TI, 960 ms; FOV, 24 × 24 cm; matrix size, 320 × 320 pixels; and NEX, 1. The T2 FLAIR parameters were TR, 9600 ms; TE, 117 ms; TI, 2400 ms; FOV, 24 × 24 cm; matrix size, 288 × 256 pixels; and NEX, 2.
Parameters for DW-MRS were TR, 2000 ms; TE, 144 ms; NEX, 8; elected volume size, 2 × 2 × 2 cm3 (8 mL); spectral width, 5000 Hz; data points, 4096; and b factors, 45 and 1050 s/mm2.
Global shimming was performed with a standard nonselective shimming sequence. Then local shimming in the selected voxel was required to obtain a water full width at half maximum of 3–5 Hz. The total measurement time for a set of DW-MRS series was 8 minutes 24 seconds.
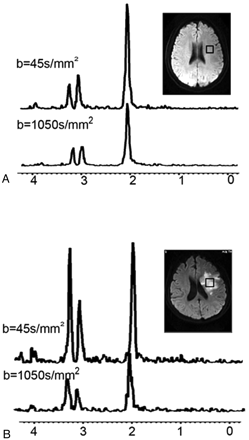
For both the healthy controls and patients with ischemia, the voxel location for MRS was set at the left centrum semiovale (Fig 2). The voxel size remained constant for each patient, even though the lesion sizes may have varied.
Brain regions. A, Brain regions of volunteers. The volume of interest (2.0 × 2.0 × 2.0 = 8 cm3) is located in the left centrum semiovale, and the spectrum is taken at low (45 s/mm2) and high (1050 s/mm2) b-values. B, Brain regions of patients. The volume of interest (2.0 × 2.0 × 2.0 = 8 cm3) is located in the region of infarct, and the spectrum is taken at low (45 s/mm2) and high (1050 s/mm2) b-values.
Data Processing
Postspectral processing was completed with SAGE software (GE Healthcare). To reduce residual water from each suppressed frame, pure water subtraction was used. Because FID varies with phase shift, the phase correction of individual data traces was used to restore phase coherence and avoid signal-intensity loss before the summation of FIDs.10 Also, fast Fourier transformation and Gaussian line-shape fitting in the automated macroprogram were applied to avoid operator bias. The automatic phase algorithm used a simplex optimization process to maximize the sum of the real components of the complex dataset. The 3 metabolites examined in this study were NAA (2.02 ppm), Cr (3.03 ppm), and Cho (3.22 ppm). NAA is the second most abundant metabolite in the human central nervous system. It is located predominantly in axons and neurons.28 Cr and Cho are the major metabolites that can be detected in the brain. They can be found in all types of cells in the brain.29 Because the integral peak area was more sensitive to the random noise,30,31 peak height was used to determine the signal intensity of metabolites in this study.
The ADC was calculated by the following equation:
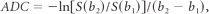
where S (b1), and S (b2) are the signal intensities for the 2 b-values, b1 and b2.
Statistical Analysis
Descriptive statistics were used to summarize subject demographics. Potential group differences in age were examined with 1-way analysis of variance (healthy older controls and patients with acute and subacute ischemia).
Student t tests (2-sided, unequal variance) were used to compare metabolite ADCs between younger and older healthy controls. To examine the effects of the duration since the onset of cerebral ischemia on the diffusion of the metabolites, 2-way analysis of variance was performed with least significance difference post hoc analysis where necessary. The level of significance was set to P < .05 for all the above tests.
Results
Age was not significantly different between the healthy older, acute, and subacute ischemia groups (P = .76). Furthermore, the number of men and women was also similar between the 2 healthy groups (P = .35).
ADCs in Aged and Young Healthy Volunteers
The ADC values of the metabolites NAA, Cr, and Cho were less in the healthy older control group compared with the younger group (P = .000052, 0.000018, and 0.00075, respectively). Specifically, values were approximately 27%, 32%, and 26% lower, respectively. Water ADC of young healthy volunteers was slightly lower than that of the older control group with no significance (Table 2).
ADCs of metabolites and water in the left centrum semiovale of healthy volunteers and patients with ischemia at different ages
ADCs in Patients with Acute and Subacute Cerebral Ischemia and Age-Matched Controls
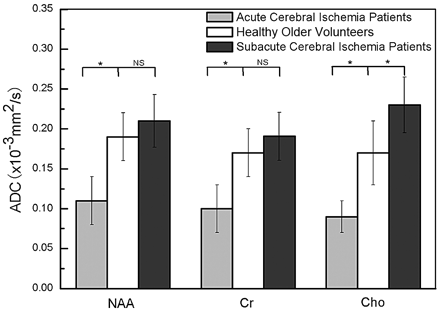
The ADC values of NAA (F2,36 = 6.057, P = .006), Cr (F2,36 = 5.634, P = .008), and Cho (F2,36 = 8.167, P = .001) were significantly different among the acute cerebral ischemia group, subacute cerebral ischemia group, and healthy controls. Post hoc testing revealed that the ADCs of metabolites obtained from patients with acute ischemia were decreased compared with those obtained from healthy older controls (pNAA = 0.023, pCr = 0.032 and pCho = 0.048). Meanwhile, the ADCs of metabolites in patients with subacute cerebral ischemia showed a higher increase in NAA, Cr, and Cho compared with those in the healthy older controls (Fig 3). Sex effects were not observed for any ADC values of metabolites. Water ADC values of patients with acute cerebral ischemia were lower than those of the patients with subacute cerebral ischemia (Table 2).
Mean metabolite ADCs for regions of interest across subjects with acute cerebral ischemia and subacute cerebral ischemia and the healthy older volunteer group, respectively. Gray bars show ADC values for the acute cerebral ischemia group, white bars show ADC values for the healthy older volunteer group, and black bars are used for the subacute cerebral ischemia group. Standard error bars and significance are also shown. Statistically significant differences relative to controls: the asterisk indicates P < .05; NS, no significance.
Discussion
The ADC values of the major cerebral metabolites NAA, Cr, and Cho were lower in older compared with younger healthy adults. Meanwhile, ADCs of these metabolites were affected by the duration from the onset of cerebral ischemia. Compared with healthy age-matched controls, metabolite ADCs were decreased in the acute stage yet increased in the subacute stage. The results of water ADC were consistent with those in previous work,32⇓–34 which will not be discussed further.
The ADCs of the 3 metabolites in the young healthy subjects were in agreement with previous reports.10,14,35,36 These results confirm that the techniques used in this study are valid.
It has been reported that all morphometric measures of neurons are significantly reduced during aging in terms of the total dendritic length, total dendritic surface area, total volume, dendritic spine numbers and densities, and dendritic diameter.19,22 This finding implies that these changes in dendritic morphology may decrease neuronal space for metabolite diffusion and, in turn, reduce the ADCs of the metabolites. On the other hand, the reduction of ADC values in the group of older compared with younger healthy controls may be attributable to the presence of neurofibrillary tangles, the paired helical filaments formed by abnormal components of the neuronal cytoskeleton and senile plaques in the neurons.22 Alterations in these structures may restrain the diffusion of the metabolites, leading to the reduction of the ADC of the metabolites. All the above-mentioned reasons may result in decreasing the ADCs of the metabolites in elderly individuals, compared with young subjects.
The current results indicate that aging has a significant effect on ADCs, which suggests that this variable should be considered when examining cohorts of different ages. In this study, it was found that the ADCs of patients with subacute cerebral ischemia are higher than those in the age-matched healthy controls, whereas ADCs of patients with acute cerebral ischemia are lower than those in the same controls. It is speculated that the balanced effect of apoptosis and necrosis of cells may play an essential role in bringing down the ADC values of patients with acute ischemia compared with healthy controls.
During ischemia, the apoptosis of the neuron occurs as early as 0.5 hours and peaks between 24 and 48 hours after onset of MCA occlusion, whereas a fully mature necrotic lesion forms in cortex after 48 hours.37⇓–39 Because NAA is considered a neuronal marker, the decrease in the ADC of NAA with acute ischemia implies the change in the affected neuronal cells in vivo. Most apoptotic neurons prevail in the inner boundary zone of the infarction and appear morphologically intact and shrunken.24,40 As a result, the size of the apoptotic neurons is remarkably smaller than that of normal neurons.41 In particular, during the ischemia process, the percentage of shrunken neurons in the lesion increases and the sizes of these neurons decrease with time.41,42 Consequently, the volume of apoptotic neurons is smaller than that of the normal neuron and the space for the diffusion of metabolites is reduced in the apoptotic neuron.41 In turn, the ADCs of the metabolites may decrease in the shrunken neurons. Necrotic cell death, such as neuronal death, predominates in the ischemic core.24,40 Necrosis is characterized by a disruption of the cell membrane.23 Because of the property of metabolites, they can leak from the necrotic neurons into the extracellular space. Given that the ADCs of metabolites in the extracellular space are greater than those in the intracellular space, the necrosis of neurons will increase the ADCs of the metabolites.43 For patients with subacute ischemia, the ADC of Cho leads to a larger increase than other metabolites. Because the Cho peak is composed of several metabolites, the total ADC might come from different diffusion properties of these metabolites. Thus, further investigation is needed before any conclusions can be drawn.
Some literature has reported that in the occlusion of the MCA for animals with different age groups, more extensive cortical infarctions were observed in the aged group than in adults.44 It was also found that older animals show more neuronal damage than their younger counterparts.45 As a consequence, the metabolite ADCs of the older patients with infarction may be different from those of the younger ones. We believe this might explain the discrepancy in ADC values between published results.
Based on the aforementioned factors, the ADCs of the metabolites in the infarct are time-dependent and may be determined by the combined effects of apoptosis and necrosis. In the acute phase of the infarct, most neurons shrink due to apoptosis, while most of the necrotic neurons are intact in the core. Consequently, within 48 hours after the onset of MCA occlusion, the ADC of the metabolites is reduced.7,14,43 This is also supported by a previous animal study in the neonatal rat brain with global ischemia showing that the ischemia-induced brain metabolite decline may partly be caused by descendent diffusion displacement. It was also suggested that the well-known water ADC drop in ischemia may be partly caused by reduced diffusion displacement of intracellular water.46 In the chronic phase of ischemia, a fully mature necrotic lesion occurs in the core of the infarct and the debris of the brain cell is liquefied, which decreases the viscosity of the extracellular space. At the same time, the number of the apoptotic neurons gradually decreases and may result in an increase in the ADC of the metabolites in the infarct area.
Several limitations to our study should be emphasized. First, the sample size of our study is small, especially for the acute ischemia group. Therefore, future larger scale longitudinal studies are needed to reveal the potential physiologic and pathologic changes underlying alterations in the ADC values of major brain metabolites. Second, the aim of this study was to offer an alternative method to understanding the physiologic and pathologic changes in different stages of cerebral ischemia. Thus, it will be more attractive if we can acquire the ADCs of the metabolites in the first 24 hours after the onset of ischemia. However, we were unable to achieve this in the current work because the conditions of the patients with early infarct were unstable and patients had difficulty holding their heads still. In our future study, this issue will be solved by accelerating our pulse sequence and applying extra equipment to help the volunteers stay still during the measurement. Finally, our results indicate that the metabolite ADC varies with age group, whereas only older patients were included in this study. A separate and more extensive experiment regarding the more complicated cases of young patients with ischemia will be performed in our future work.
Conclusions
This study shows that the diffusivity values of intracellular metabolites NAA, Cr, and Cho are more significantly decreased in healthy elder volunteers than in healthy young volunteers. Detailed analysis of diffusion changes experienced by the 3 detected metabolites shows that ADCs are negatively correlated with aging. This is the first report of a completely noninvasive simultaneous assessment of the intracellular distribution of NAA, Cr, and Cho by considering the age effect in humans in vivo. The ADCs of metabolites in patients with subacute cerebral ischemia are higher compared with the age-matched healthy controls, whereas the ADCs of metabolites in patients with acute cerebral ischemia are lower compared with the same controls. These findings give support to the pathologic changes in the intracellular environment at different stages of cerebral ischemia in vivo. These results also indicate that the effect of aging should be considered when studying the role of brain metabolite diffusion in the ischemic intracellular environment at different stages by using DW-MRS.
Acknowledgments
We thank Brad Manor, PhD, and Hua Zhong, PhD, for their help in revising the manuscript.
Footnotes
-
Jue Zhang and Xiao Ying Wang received research support (including provision of equipment or materials) from the Fundamental Research Funds for the Central Universities of China (2009-II-006).
-
Paper presented in part as a poster at: 19th Annual Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine, May 6–13, 2011; Montréal, Québec, Canada.
References
- Received April 16, 2011.
- Accepted after revision June 16, 2011.
- © 2012 by American Journal of Neuroradiology