Abstract
SUMMARY: Intracranial hypotension is a rare cause of persistent headache mostly originating from a dural CSF leak. If a conservative treatment fails, a minimally invasive EBP can lead to a successful sealing of such a leak. Independent of the leakage site, an EBP is usually applied at the lumbar level with varying success. We used CT myelography to detect the site of the dural leakage, then immediately applied a targeted EBP at the corresponding level to patch the leak. Seven patients from our clinic were treated with a single targeted EBP in the lumbar or cervical spine. Within 24 hours, 6 patients experienced a considerable relief of symptoms; 1 patient went into remission after a repeat procedure. Our preliminary data suggest that a CT-guided, CT myelography-assisted targeted EBP is a safe and effective treatment for persistent spinal CSF leaks.
ABBREVIATIONS:
- EBP
- epidural blood patch
- IHS
- intracranial hypotension syndrome
- LP
- lumbar puncture
- SDH
- subdural hematoma
IHS usually manifests with symptoms including orthostatic headache, nausea, neck pain, vomiting, and dizziness. In most cases, a persistent CSF leak is the cause of IHS. Such a persistent dural leak may sometimes arise spontaneously due to a rupture of subarachnoid perineural cysts.1 However, it occurs most commonly as an undesirable effect of a lumbar puncture or is related to trauma or spinal surgery.2
Patients frequently recover spontaneously; therefore, the first approach should be conservative treatment including bed rest, analgesic medication, and oral hydration to restore the depleted CSF volume. If the conservative treatment fails, an EBP should be considered as a minimally invasive therapy option before taking surgical intervention into account. In general, the EBP itself can be performed either blindly at the lumbar level, or it may be targeted at the suspected leakage site. Most studies, however, arrive at the conclusion that a blind lumbar EBP is preferred due to an assumed lower risk, though a targeted EBP appears to be more effective.3⇓⇓⇓⇓⇓⇓–10 Thus, only a few reports of targeted EBPs exist in the literature.11⇓⇓⇓–15
In contrast to a blind EBP, a targeted EBP relies on an accurate localization of the leakage site. To this end, several imaging techniques are available, including CT myelography, radioisotope cisternography, and MR imaging of the spine. However, even if the site of the leak is known precisely, the execution of the CT-guided targeted patch is difficult due to the limited anatomic resolution of the planning CT scan for intra- and extradural compartments.
In view of these problems, we performed CT myelography in a group of 7 patients with IHS and proved epidural CSF leak. We then used the resultant contrast-enhanced images to plan, and also guide, the targeted EBPs.
Materials and Methods
After giving informed consent, all patients underwent a CT myelography with subsequently guided autologous epidural blood patches at the site of the leak. The myelography consisted of an injection of 15 mL of contrast agent (Solutrast 250 mol/L; Bracco, Konstanz, Germany) into the subarachnoid space between the lumbar vertebrae L3/L4 or L4/L5, after which the patients had to assume a horizontal position for 30 minutes, while turning from side to side every 3 minutes to guarantee adequate distribution of contrast medium along the whole spinal canal. Subsequently, a CT scan of the whole spine was performed (Brilliance 64 [Phillips, Best, the Netherlands], 120kV, mean 223mAs, mean effective radiation dose 14mSv) by using sagittal and coronal reconstructions to improve the diagnostic accuracy. A leakage site could be found in all patients. The mean time between the CT myelogram and the EBP was 3 hours (SD ± 2 hours). For the EBP, patients were positioned face down in the CT scanner. To take blood, an 18G intravenous catheter was placed in a brachial vein. Thereafter, a planning CT scan (50 mAs, 120 kV) was performed in the volume of interest.
We used intermittent CT fluoroscopy with a low-milliampere technique to place a 20G coaxial needle (Quincke bevel, Spinocan; Braun, Melsungen, Germany) in the epidural space at the suspected leakage site under strict aseptic conditions.
Contrast-enhanced CSF in the epidural space helped to position the needle tip safely and with high precision.
Blood was drawn from a peripheral intravenous catheter (6–20 mL, mean 15 mL) and mixed with 1 mL of contrast medium (Solutrast 250 mol/L; Bracco). This mixture was then injected in the epidural space until the patient reported a local pressure sensation or commencing pain. A final CT scan of the leakage zone was performed to assess the correct epidural spread of blood-contrast mixture. Following this procedure, patients were advised to remain in a horizontal position for at least 6 hours.
Results
We retrospectively reviewed 7 patients (5 women and 2 men; age 31–61; mean age 40) who were admitted to our hospital between October 2009 and February 2011, initially presenting with orthostatic headache (n = 7), nausea (n = 2), diplopia (n = 1), dizziness (n = 3), or hearing loss (n = 1). Detailed patient data are displayed in the Table.
Patient characteristics
In 3 patients, we could detect a temporal association between appearance of symptoms and a spinal intervention (facet joint injection, n = 2; peridural nerve therapy, n = 1). The remaining patients had no history of trauma.
All patients had received a CT or a MR imaging of the brain. In 2 patients the performed CT showed no IHS-specific findings. Five patients underwent cerebral MR imaging; 2 of these showed typical findings of low intracranial CSF pressure. These findings consisted of subdural fluid collection as well as pachymeningeal thickening, combined with diffuse pachymeningeal contrast enhancement. In 1 case, the MR imaging revealed unilateral chronic SDH. Additionally, 1 patient showed venous engorgement (left transverse sinus, bilateral vena cerebri media). In 2 patients, no abnormal brain MR imaging findings could be observed.
To detect the CSF leak, a spinal MR imaging was performed in 4 patients, including sagittal and axial fat-suppressed T2-weighted sequences as well as contrast-enhanced sagittal T1-weighted sequences. One patient showed a mild form of abnormal engorgement of the spinal epidural venous plexus at the upper thoracic level. However, the exact localization of the dural leakage could not be identified. For 3 patients, the MR imaging of the spine was interpreted as normal.
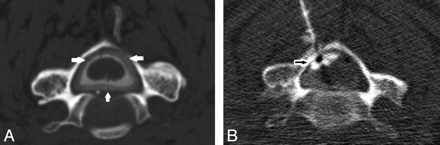
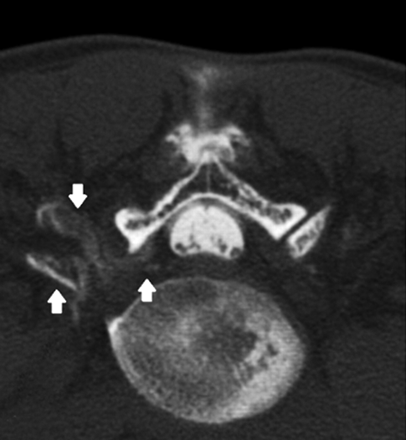
Contrary to spinal MR imaging, CT myelography detected abnormal findings in all patients. In 5 patients, it revealed an accumulation of contrast medium in the epidural space, with maximum intensity in the cervical (n = 3) or lumbar level (n = 2), as shown in Fig 1 for a representative case. Two patients had a diffuse extradural, bilateral spreading of contrast medium along nerve root sleeve L3 and L5, respectively (Fig 2).
A, Example of a postmyelography CT revealing a cervical dural leak with circular accumulation of contrast medium in the spinal epidural space (white arrows). B, Targeted EBP in the same patient with epidural spreading of the blood-contrast mixture (unfilled white arrows).
Postmyelography CT showing an unilateral extradural spreading of contrast medium around the right nerve root L4 (white arrows).
Before receiving a targeted EBP, conservative therapy such as bed rest, overhydration, and intake of analgesic drugs over 2 days to 4 weeks (median 10 days) had failed in all patients. After the EBP, 6 patients reported a considerable relief of symptoms within 24 hours and could be released from the hospital in 2 to 6 days (median 4 days) after the procedure. One patient with a genetic disorder of the connective tissue and joint hyperflexibility had a relapse of symptoms after 2 days. She underwent a second EBP at a further suspected leakage site and experienced complete recovery within 24 hours. No complications were observed in any of our patients.
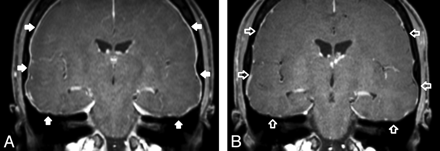
One patient with initially prominent dural contrast enhancement showed complete remission on an MR imaging of the brain 2 months after the intervention (Fig 3).
A, Intracranial pachymeningeal thickening and contrast enhancement in a 31-year-old woman with symptoms of IHS (white arrows). B, Renormalization of the epidural enhancement 2 months after performing a targeted EBP (unfilled white arrows).
Discussion
IHS as a cause of persistent daily headaches has been shown not to be as rare as was previously thought. The annual incidence has been estimated as 5 per 100,000.16 Orthostatic headache is the most common, but not mandatory, manifestation of IHS, accompanied by a wide variety of other symptoms such as neck pain or stiffness, nausea, hearing loss, visual blurring, facial numbness, Parkinsonism, ataxia, or dementia.2,9,17 It seems plausible that a low CSF volume due to a persistent CSF leak is the cause of this syndrome. Most often, such a leak is due to dural puncture for diagnostic LP or spinal anesthesia.18 Nevertheless, a dural leak could also be associated with dural injury in the course of spinal surgery or craniospinal trauma. In 2 of our patients, lumbar facet joint injections were practiced before the symptoms of IHS occurred. There are no reports that document facet injection as a potential cause of CSF leakage. However, in both patients with facet joint injection, CT myelography revealed diffuse extradural spreading of contrast medium at the levels treated. We had no data that documented whether the facet joint injection was performed by using palpation or fluoroscopic guidance. A violation of the dural sac during a blind procedure is rare but should be taken into consideration. In 4 patients, no cause for the existence of a CSF leakage could be found. In these cases, CSF leaks were suspected to have occurred spontaneously. There is strong clinical evidence that a significant number of these spontaneous dural leaks are related to connective tissue disorders, as seen in hereditary syndromes like Marfan syndrome and Ehlers-Danlos syndrome.19,20 In these patients, a great variety of structural abnormalities causing spontaneous spinal CSF leaks may be found during surgery. Meningeal diverticula, dural holes, dural tears, or missing nerve root sleeves have been reported.1,18
The standard procedure for the diagnosis of IHS includes imaging of the brain and spine. As far as brain imaging is concerned, MR imaging is the preferred technique, showing pachymeningeal thickening and gadolinium enhancement, engorgement of venous structures, pituitary hyperaemia, and sagging of the brain.2,21,22 These findings seem to be part of the compensation mechanism related to the loss of CSF volume.23 MR imaging of the spine has attracted less interest, mainly because of the limited ability to localize a dural leakage site.9 MR myelography, in particular, has revealed promising results,24,25 but only some institutions are performing it for diagnostic purposes; thus, the safety of intrathecal gadolinium application is yet unclear.26 Therefore, CT myelography is still the diagnostic tool of choice, as it can show the location and extent of CSF leaks with high accuracy.9,11 The de facto complication rate associated with lumbar puncture is very low and includes pain, paresthesias, meningitis, cauda equina syndrome, and persistent leakage of CSF.27 On the other hand, an untreated, persistent CSF leak may have severe consequences such as subdural hematoma, downward displacement of the brain, and nerve injury.2
Consistent with other studies, we could not detect the CSF leak conclusively by MR imaging in any of our patients. However, CT myelography revealed a dural leak in all 7 patients, with cervical localization for 3 patients and lumbar localization for 4 patients. Two patients with a lumbar leak showed nerve-root-associated spreading of contrast-enhanced CSF, thus implicating a lacking or damaged nerve root sleeve at this level.
It is believed that most dural leaks occlude spontaneously within a few weeks. Therefore, conservative measures should be sought at first, including bed rest, oral hydration, caffeine intake, and the use of an abdominal binder. If symptoms persist or even aggravate, then further, more invasive therapeutic options should be taken into account.
EBP is the most common therapy in patients with a persistent CSF leak, though it has been questioned, as a systematic review has revealed less favorable results than initial reports suggested.28
Although CSF leaks occur most often in the cervical or thoracic spine, an EBP is commonly applied without visual control at the lumbar level and is not performed at the suspected site of the leak. However, to ascertain distribution of blood to the upper spine, the patient needs to be seated in a Trendelenburg position after the injection.9,29 There are a few reports that observed a minor upward spread of a lumbar blood injection by up to 4–6 spinal segments.3,8,29 However, when blood is injected at a site distant from the CSF leak, it is difficult to conceive how the blood can traverse to the desired, remote location, with clotting commencing immediately after injection. Despite some reports of successfully treated CSF leaks with this method, animal experiments have shown that an EBP performed directly at the site of the leak will seal the dural defect more effectively than a blind epidural patch in the lumbar region.10 This suggests that, for therapeutic purposes, the local sealing effect of the epidural tamponade is the predominant effect.9 Some reports state a mean injected blood volume of about 30 mL, and up to 100 mL in certain cases.9,29 Conversely, a targeted blood patch requires much lower amounts of blood (mean 15 mL) and thereby reduces the chance of complications.
Despite these facts, the blind lumbar patch is still preferred due to a higher risk of spinal cord violation during cervical EBP. To minimize this risk and to decrease the rate of symptom relapse, we performed CT myelography followed immediately by a targeted EBP. This way, the contrast enhancement of the spinal intra- and extradural space is used to initially diagnose and to subsequently guide the EBP safely, due to the positively identified epidural space (as seen in Fig 1). Recently, Kranz et al14 also reported a series of 8 patients who were treated with targeted epidural blood patches by using a similar procedure with promising results. In contrast to our method, in which only a single EBP is applied, they used several blood patches in each patient, ranging from 1–10 in the initial treatment session.
The loss of resistance puncture technique is usually applied to inject medication into the epidural space, in particular by anesthesiologists.30 However, the loss of resistance due to loosely packed tissue in the epidural space (containing fat, veins, and emerging nerve roots of the spinal cord) cannot be detected reliably if the epidural space is filled with CSF caused by a dural leak.27 Therefore, contrast-enhanced CT images could help to place the needle tip in the epidural space exactly, without the risk of damaging the spinal cord. Furthermore, our method could improve EBPs, even in case of a lumbar leak, because the injected amount of blood could consequently be minimized without reduction of the tamponade effect.13 In addition, the risk of subarachnoid blood injection resulting in an intrathecal hematoma, accompanied by severe back pain and radicular symptoms, could be reduced.29
For the 7 patients who we have reviewed in this study, there were no complications or adverse events. Six out of the 7 patients experienced a considerable relief of symptoms within 24 hours after the procedure. The one remaining patient went into remission after a repeat EBP at an additional suspected leakage site.
We are well aware of the limitations of this study. First, as already criticized in other studies and as per definition of this kind of study, there is no control for spontaneous remission in our patients.
Second, the inclusion of more patients would yield better data, in particular, regarding efficacy and safety of our procedures. However, the extra radiation dose that is incurred by doing a large CT myelogram study is a limiting factor because the reduction of radiation is an important aim. Although a CT myelography-guided, targeted EBP seems effective and low risk, it might not be necessary if medical history and diagnostic findings clearly indicate a spinal CSF leakage. In this case, an initial blind lumbar EBP may reduce radiation dose. If this fails, a CT myelography-guided targeted EBP could be the next step of escalation.
In the case of lumbar CSF leaks, a blind EBP is certainly a possible treatment option. However, if myelography is performed for diagnostic purposes, an immediate targeted patch can probably seal the leak more effectively and safely.
Conclusions
We performed single CT myelography-guided targeted epidural blood patches in 7 patients with symptoms of IHS and positive imaging findings. Our preliminary data reveals this minimally invasive method to be safe and effective for the treatment of patients with persistent spontaneous or traumatic spinal CSF leakages.
References
- Received April 11, 2011.
- Accepted after revision June 6, 2011.
- © 2012 by American Journal of Neuroradiology