Abstract
BACKGROUND AND PURPOSE: Interspinous spacers are implanted to treat symptomatic lumbar stenosis. Posterior vertebral element fractures can occur during or after interspinous spacer implants, especially in patients with osteopenia. The purpose of our study was to assess the biomechanical rationale, safety, feasibility, and effectiveness of posterior vertebral arch cement augmentation (spinoplasty) in preventing delayed spinous process fractures after interspinous spacer implants in patients with risk factors for fragility fractures.
MATERIALS AND METHODS: We performed a nonrandomized historically controlled clinical trial. From June 2007 to March 2010, we implanted interspinous spacers in 35 eligible patients with fragility-fracture risk factors. In 19/35 patients treated after April 2009, after we assessed the theoretic biomechanical effects of cement augmentation of the spinous process and laminae by FEM, a percutaneous spinoplasty was also performed. Clinical and radiologic follow-up ranged between 12 and 36 months after the intervention.
RESULTS: No intraprocedural spinous process fractures were observed in either group, and no patients in the 24-hour postoperative period had complications that were procedure-related. Symptomatic delayed spinous process fractures were diagnosed in 4/16 patients who did not undergo spinoplasty (25.0%), while no fractures were diagnosed in the 19 treated patients (P = .035).
CONCLUSIONS: Spinoplasty is feasible and safe. It has a biomechanical rationale, as demonstrated by an FEM. In our preliminary experience, it seems effective in preventing delayed fractures of the posterior arch post-interspinous spacer placement in patients at risk for fragility fractures. These patients have a significant risk of developing a symptomatic delayed spinous process fracture if not treated with spinoplasty.
ABBREVIATIONS:
- AP
- anteroposterior
- FEM
- finite element method/analysis
- FSU
- functional spinal unit
- INC
- intermittent neurogenic claudication
- IQR
- interquartile range
- LL
- laterolateral
- PMMA
- polymethylmethacrylate
INC is the typical clinical manifestation of lumbosacral nerve root compression. It is characterized by weakness, discomfort, pain, or altered sensation, radiating to the buttocks and lower limbs, initiated by prolonged standing or walking, exacerbated by lumbar extension, and improved by lumbar flexion.1⇓–3 Nerve root compression is mostly a consequence of degenerative canal and foraminal stenosis in middle-aged and elderly patients. A multicenter prospective trial demonstrated the efficacy of an interspinous spacer device (X-STOP, Medtronic, Minneapolis, Minnesota)4⇓–6 in treating such a condition. Additional interspinous spacer devices were subsequently proposed on the basis of the same principles.7⇓⇓–10 A possible complication of such devices is the intraprocedural or delayed fracture of the spinous process and/or laminae adjacent to the device.6,11 Consequently, this treatment is contraindicated in patients with osteoporosis.4⇓–6,9,10,12 Additionally, due to the frequent bone mass loss, advanced age (older than 75 years) can be considered a risk factor as well.
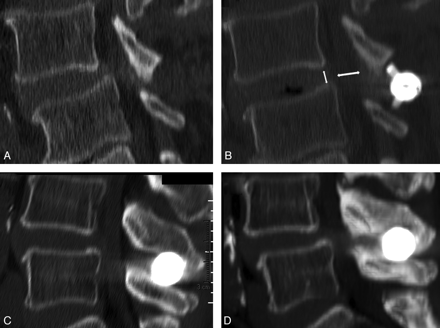
PMMA bone cement augmentation of the posterior vertebral elements has been proved to increase stiffness and failure load values of the augmented bony structures in a postmortem study.9 At our center, since June 2007, we have been treating eligible patients with INC by implanting interspinous spacers and have been encountering symptomatic postimplantation delayed fractures (Fig 1).
A and B, Reformatted sagittal CT images of the lumbar spine show central canal stenosis and spondylolisthesis (A), partially corrected after interspinous spacer implant (B). C and D, In a different patient, postimplantation CT (C) and follow-up CT (D), prompted by neurogenic claudication recurrence, show support failure of the spinous processes and subsidence of the implant, with recurrence of central canal stenosis.
The purposes of this study were the following: to assess the biomechanical rationale of cement augmentation of the posterior vertebral arch (spinoplasty) by an FEM, to describe the technique of spinoplasty associated with interspinous spacer implants, to assess its feasibility and safety in patients with risk factors for fragility fractures, and to evaluate preliminary results on effectiveness in preventing delayed fractures of the spinous processes in these patients.
Materials and Methods
Preliminary FEM
Preliminary to the beginning of the clinical study, we conducted an analysis simulating intraspinous and intralaminar cement injection by using the FEM.
Osteoporosis was simulated in the L1-L2 spinous processes as well as in the L1-L2 laminae.
The lower vertebra (L2) was not allowed to move in any direction. With the mid-disk plane oriented horizontally, a compressive load of 450 N was applied to the upper vertebra (L1) so that all nodes of the uppermost plane of L1 were loaded.
Although the most commonly affected and treated levels are L4-L5, we used an L1-L2 model. The force applied to the lumbar vertebra L1 represents the value of the load that usually affects the lumbar vertebra below (L4), equivalent to a 70-kg person, taking into account body weight, including trunk, head, and arms. To produce the correct loading condition of the level of L4-L5, we finally applied the angulations of the vertebral bodies L4-L5 to the model.
By using this configuration, a proportionately smaller vertebra is loaded with a compressive force, which is greater than the force usually experienced by the L1 vertebra. This loading condition was used to introduce an ideal safety factor in our calculations.
In this scenario, bone cement was added to strengthen the spinal levels involved by osteoporosis. Further analysis was conducted to assess the final effect of bone cement on stress and deformation of a titanium ring located between the above-mentioned spinous processes. The elastic modulus of bone cement was considered to be an intermediate value between the cortical bone and the cancellous bone. The characteristics of the material were simulated as follows—Titanium ring: Young modulus = 106 GPa, Poisson ratio = 0.34; bone cement: Young modulus = 2 GPa, Poisson ratio = 0.3. Loading and constraint configuration were as follows—Loading conditions: uniform pressure distributed on the upper vertebral plate (L1); constraints: the nodes lying on the L2 lower vertebral plate rigidly fixed (zero df) to simulate the vertebral plate lying on a plane.
Data Collection and Patients
From June 2007 to March 2010, we performed, at a single center, interspinous spacer implantation (Aperius PercLID, Medtronic)3,4,8⇓–10,13,14 in all eligible patients with INC and radiologic diagnosis of central canal and/or foraminal stenosis. All patients underwent an MR imaging or CT study of the lumbar spine preoperatively, to confirm stenosis of the central canal and/or foramina and to diagnose the most severely affected levels. Correct implantation of the interspinous device, absence of intraprocedural fractures of the spinous processes, and other technical complications were ruled out by means of an x-ray study immediately after the intervention or, in doubtful cases, with a postoperative nonenhanced CT study of the lumbar spine, the same day of the procedure. One experienced spine interventional neuroradiologist (first author) performed all the procedures.
A clinical follow-up was performed in all cases, with duration between 12 and 36 months. Patients with new-onset low back pain and/or recurrent INC symptoms during the follow-up period underwent an MR imaging and/or CT study of the lumbar spine.
Patients were classified at risk of fragility fractures using 1 of the following criteria: age older than 75 years, osteopenia (as defined by World Health Organization on a bone scan with a t-score < −1.0), osteoporosis (t-score < −2.5), history of prior fragility fracture, and chronic steroid therapy. From May 2009 to March 2010, all patients with ≥1 risk factor for fragility fractures underwent prophylactic spinoplasty at the levels adjacent to the interspinous device during the same procedure and just before the interspinous spacer implantation. In addition to the clinical follow-up, ranging between 12 and 22 months, these patients also underwent a lumbar spine LL plain film or CT examination to assess posterior arch fracture, device dislodgment, or other possible technical complications 3 months after the procedure. The study was approved by our local institutional review board. All patients were informed of the investigational use of prophylactic spinoplasty and of the off-label use of the interspinous spacer device in osteoporosis and signed an informed consent.
Surgical Technique
The procedures were performed in the angiography suite with biplane fluoroscopy, with the patient under local anesthesia and moderate conscious sedation.
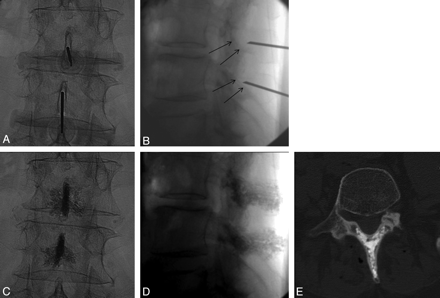
For posterior arch cement augmentation, we inserted a 15-ga 10-cm vertebroplasty needle into each spinous process above and below the level of interspinous spacer placement using a posterior midline approach (Fig 2). Progression of the needle, obtained through a mallet, was controlled in alternating biplane fluoroscopy. In the LL view, the tip of the needle was advanced along the center of the spinous process, at mid-distance from the caudocranial height or, alternatively, closer to the opposed aspects of the spinous processes adjacent to the interspinous space in which the spacer device was to be inserted. We advanced the needle to the spinolaminar junction, paying attention not to perforate the ventral cortex of the lamina. In this regard, the fluoroscopic landmark of the dorsal aspect of the inferior articular process can be considered a safe limit (Fig 2B). In the AP view, the needle was carefully maintained at the same distance between the 2 lateral cortical surfaces, because the spinous processes are thicker posteriorly than in their middle third, where they can become very thin. For these reasons, we used beveled-tip needles, which can be steered on the basis of the side of the bevel surface. This feature also helps in controlling the craniocaudal angle of progression.
A and B, Intraprocedural fluoroscopic images show needle placement in the spinous processes for spinoplasty. The fluoroscopic ventral limit to avoid central canal violation is the posterior margin of the inferior articular process (black arrows on B). C–E, Postspinoplasty fluoroscopic (C and D) and CT (E) images show PMMA distribution in spinous processes and laminae.
Careful and slow injection of regular vertebroplasty PMMA cement (HV-R; Kyphon, Sunnyvale, California) was performed with 1-mL syringes, under continuous LL and intermittent AP fluoroscopic control. Given the reduced thickness of the spinous process and its cortical layer, compared with the vertebral body, we tended to inject cement in a less viscous phase than usually recommended for vertebroplasty. When necessary, the needle was slightly repositioned to ensure satisfactory filling of the medullary cavity of the spinous process close to the cortex. Despite this, only rare and minor venous leakages were observed. Cement was injected to fill at least the ventral two-thirds of the spinous process and the proximal more medial parts of the laminae (Fig 2C–E). Usually 1 to 2 mL of cement per level were sufficient.
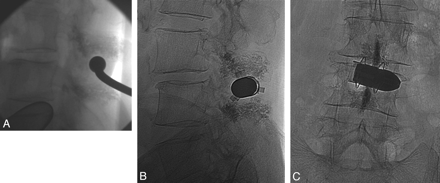
During injection, the needle was progressively withdrawn to fill the dorsal components of the spinous process. Extraosseous leakage (see the “Results” section) was easily recognizable on intraoperative fluoroscopic images due to the more compact aspect and better defined borders, compared with the proper intraspongious cement. If extraosseous leaks were observed, we stopped injection for 8–10 minutes. After hardening of the leaking cement and closing of the leaking fissure, it became possible to safely resume the injection. The spinoplasty portion of the procedure usually required 10–15 minutes overall. An interval of approximately 10 minutes was observed before performing Aperius implantation to allow the PMMA to harden in the bone. The Aperius device was implanted by using the standard percutaneous technique (Fig 3). The details of this technique are beyond the interest of this article.
Intraprocedural fluoroscopic image (A) of the percutaneous insertion of the interspinous spacer, following spinoplasty. Postimplantation control images (B and C) show the end result of spinoplasty and interspinous spacer implant.
Statistical Analysis
Proportion was used as a descriptive statistic for categoric and ordinal variables: median and IQR for ordinal variables; median, IQR, mean, and SD for continuous variables. The occurrence of either intraprocedural or delayed fracture of the spinous processes and/or the laminae adjacent to the device was analyzed in 2 different between-group comparisons: 1) patients without risk factors for fragility fractures treated between June 2007 and April 2009 versus patients with risk factors treated in the same period, and 2) patients with risk factors treated between June 2007 and April 2009 versus those with risk factors treated between May 2009 and March 2010. The first analysis was designed to validate the prognostic value of the identified risk factors and served to confirm the eligibility criteria to perform the prophylactic spinoplasty in the subsequent patients. The second analysis was aimed at assessing the effectiveness of the spinoplasty in preventing fractures. In both cases, the occurrence of fractures was compared by using the 2-tailed Fisher exact test, with .05 as level of significance. Data were analyzed by using SAS software (Version 9.1.3; SAS, Cary, North Carolina).
Results
FEM
The FEM analysis was developed on the FSU in 3 different situations: osteoporosis in the spinous processes and laminae (case 1), bone cement in the spinous processes and osteoporosis in the laminae (case 2), and bone cement in the spinous processes and laminae (case 3).
Compared with the osteoporosis FSU, the bone tissue stiffness increased by 116% due to the presence of bone cement.
Analysis confirmed the mechanical standard behavior: opposite proportion correlates with the spinous processes bending modulus and spinous processes deflection, and the deflection of this process results in a different compression of the metal ring.
In case 1, in which general osteoporosis affected both processes and laminae, the bending modulus was the lowest. In cases 2 and 3, injection of bone cement in the spinous processes (case 2) and both spinous processes and laminae (case 3) were simulated. In such conditions, the increase in stiffness and the higher modulus generated in the posterior arch produced the reduction of values of stress and strain quantified in the Table.
Finite Element Analysis
Compared with case 1, the stress of the model in case 2 and case 3, respectively, decreased by 5.5% and 9.6%. A decrease of 4.3% of the strain distribution for case 2 and of 16.0% for case 3 was calculated on average.
Clinical Study
From June 2007 to April 2009, 32 patients with no known risk factors for fragility fractures (15 men; mean age, 65.8 years) were treated at 43 levels with implantation of an interspinous spacer alone (without spinoplasty). In the same period, 16 patients at risk of fragility fracture (7 men; mean age, 75.9 years) were treated with the same approach at 20 levels. No intraprocedural or delayed fractures were observed in either group, and no patient in the 24-hour postoperative observation period had any symptoms possibly related to injection of cement. Moderate back pain was always present, similar in characteristics and intensity to what was usually observed in Aperius cases.
During the 12–36 month clinical follow-up, no events were observed in the 32 patients without risk factors, while a symptomatic delayed spinous process fracture was diagnosed in 25.0% (4 of 16) of patients at risk (P = .0094). These 4 patients did not have a definite diagnosis of osteoporosis but were older than 75 years (2 patients) or had true osteopenia (2 patients, 62 and 68 years of age). The fractures occurred, respectively, at 4, 4, 5, and 6 weeks after the implant. All fractures were diagnosed at follow-up CT imaging, prompted by abrupt recurrence of INC symptoms after postoperative amelioration. In all cases, CT showed a lytic subsidence of the bone in contact with the interspinous implant device and consequent embedding of the latter into the spinous process, therefore featuring a sort of compression fracture of the spinous process rather than a true fracture.
From May 2009 to March 2010, nineteen patients at risk for fragility fractures were treated. They underwent the same interspinous spacer implant at 24 levels but also underwent a prophylactic spinoplasty at the levels adjacent to the interspinous device. The combined procedure was uneventful in all 19 patients. Neither intraprocedural fluoroscopic images nor postoperative CT scans showed intraforaminal or intravascular leakage. In 1 patient, tiny paralaminar midline posterior epidural leakage was observed. In another patient, a paraspinous extracanalar moderate leakage was detected. Both were asymptomatic. Spinal imaging follow-up 3 months after the procedure showed absence of fractures of the spinous processes or laminae, neither as lytic subsidence with Aperius embedding into the cancellous bone of the process nor as true linear fractures with bone fragment dislodgment. Metallic artifacts obscure the contact surface between the device and bone, but usually a thin fatigue cortical erosion can be suspected.
The absence of fracture in the 19 patients at risk treated with spinoplasty differed statistically from the 25.0% fracture rate in the historical control group (P = .035).
Discussion
This study describes the spinoplasty procedure technique during interspinous spacer placement and assesses its biomechanical rationale, safety, feasibility, and effectiveness in preventing delayed spinous process fractures in patients affected by INC, with risk factors for fragility fractures.
Rigid interspinous spacers were developed and validated for treatment of symptoms of INC.4⇓–6 Use of these devices is contraindicated in patients with osteoporosis, as defined by a t-score of <2.5, or with a history of prior fragility fracture.4⇓–6,9,10,12 Elderly individuals are often affected by bone fragility even without a definite diagnosis of osteoporosis. Hence, they should be considered at risk for this procedure.
We have been using the Aperius interspinous spacer device since June 2007. The Aperius is introduced through a percutaneous fluoroscopically guided approach.
After the first 48 cases, we observed 4 symptomatic delayed fractures of the spinous processes. The fractures occurred in patients with recognized risk factors for bone fragility without a definite diagnosis of osteoporosis. The delayed fractures presented with new onset of low back pain and/or recurrence of INC symptoms after a variable postoperative interval, ranging between 4 and 6 weeks. We then retrospectively stratified the patients on the basis of the presence of a generic risk factor of bone fragility (age older than 75 years, osteopenia, history of prior fragility fractures, and chronic steroid treatment). The occurrence of delayed fractures was significantly different in the 2 groups (P = .0094).
Bone failure after interspinous spacer placement and the consequent possible delayed fracture of the spinous processes are correlated to the mechanism of action of the device.
Rigid interspinous spacers obtain the desired effect (i.e. limiting extension, thus preventing the subsequent worsening of spinal canal and foraminal stenosis) undergoing compression during extension of the lumbar spine.13⇓⇓⇓⇓–18 At that moment, provided that the bone does not fail, the extension movement, no longer obtainable due to the presence of the interspinous spacer, is now obtained at the level of different elastic structures, particularly the anterior annulus and facets, which will move opposite of the normal direction, opening instead of closing. In such conditions, an increased load is transferred to the bony structures, with consequent risk of acute or chronic failure. Spinous process fractures following X-STOP implantation are reported in the literature in patients without osteopenia or osteoporosis.4,6,11 Aperius and X-STOP share the same biomechanical rationale and effects, and the results of our study should theoretically apply to both implants. The percutaneous approach of Aperius allows the use of light sedation and local anesthesia. Blood loss during the procedure is close to zero, and the surgical time is 10–15 minutes overall. Posterior arch augmentation, as described here, adds only 10–15 minutes to the duration of the procedure. Patients are immediately mobilized. These features are favorable in elderly patients.
Failure of the interspinous spacer, with subsequent recurrence of symptoms, may be related either to bony subsidence directly under the spacer (chronic fatigue fractures, as observed in our cases), where the metallic device comes in contact with the cortex of the spinous process (anterior third of the spinous process), or, less likely, to a true fracture at the level where the distraction load is stronger (ie, the spinolaminar junction).
When osteoporosis was simulated, larger areas of the cancellous bone were subjected to higher strains and principal strain magnitudes were increased in the trabecular bone. The FEM analysis we conducted on the functional spinal unit in 3 different conditions confirms these data, showing that the increase in stiffness leads to a decrease of stress and strains of the neural arch.
Increasing the elastic moduli of the bony structures with PMMA altered the strain distribution in the vertebra. The progressive decrease of cancellous bone porosity, which increases the cross-sectional area, and the ongoing architectural changes might explain the measured differences.
The presence of cement in the cancellous bone of the spinous process prevents the fatigue lysis, or subsidence, of the bone in contact with the metal. The lysis, as observed in our patients with fractures, leads to an embedding of the interspinous device into the cancellous bone of the spinous process. This eliminates the effects of the device on the sizes of spinal canal and foramina but also reduces the section of the spinous process resisting the bending loads applied, which could eventually lead to a true linear fracture.
A biomechanical study in postmortem lumbar vertebrae9 confirms the effectiveness of an increase in the resistance of bony structures to compression loads after posterior arch cement injection. Our prior experience with patients with trauma and cancer has demonstrated the feasibility and safety of cement injection in the posterior vertebral arch.
Consequently we started a study, with the aim of evaluating the feasibility, safety, and effectiveness of posterior vertebral arch cement augmentation in preventing delayed fracture of the vertebral posterior elements in elderly patients or those with osteopenia. After the introduction of such an intervention, we did not observe any delayed fractures in 19 patients at risk for bone fragility, confirming a beneficial effect of vertebral cement spinoplasty in patients at risk of delayed fracture.
Study Limitations
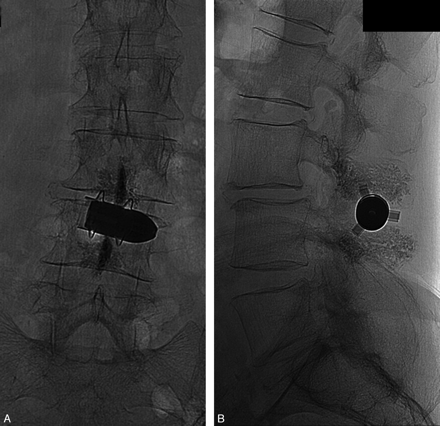
The incidence of delayed fractures in the control group may have been underestimated in this study because delayed follow-up imaging was performed only in patients with recurrence of symptoms. In contrast, delayed fractures were very unlikely to have been undetected in the patients treated with spinoplasty because they were all followed up with lumbar spine imaging 3 months after the intervention (Fig 4). In other words, if a reporting bias did exist, it acted against evidence of an effectiveness of the spinoplasty.
A and B, Radiologic follow-up 3 months postimplantation with plain films in 2 views of the lumbar spine shows the device in place and the absence of significant subsidence or fractures of the spinous processes or laminae. Minimal cortical bone remodeling at the bone-metal interface is noted.
The minimal follow-up of 12 months for some patients might be considered too short to be conclusive because they may develop insufficiency fractures at a later date. However, all the fractures in the control group occurred between 4 to 6 weeks postoperatively.
The absence of randomization may be of concern because it does not guarantee the control for unknown confounders. Nevertheless, particularly if some conditions are satisfied, historically controlled studies can provide convincing evidence: first, temporal trends in supportive care or physician's learning curve should be excluded; second, methods of patient evaluation should be the same in the current and historical control groups; and third, the case mix should remain constant in the 2 groups. All these conditions were fulfilled in this study because the patients were treated within a short timeframe by the same already skilled staff and were followed and examined with the same approach, if not even more thorough in the study group.
Despite the statistically significant results, the small sample size requires the data to be taken with caution and calls for confirmatory evidence.
One concern could be that while the operator injects the medial part of the laminae, the cement superimposes on fluoroscopy to the spinal canal, simulating a possible epidural leakage. However, the morphology of the cement is definitely different when it remains confined to the bone (intralaminar), with the typical multicellular spongelike aspect with fuzzy borders, while extraosseous cement appears more globular, homogeneous with clearly defined margins. Of course, the topography of the cement, frequently and carefully checked, alternating AP and LL projections, also helps confirm its correct distribution during injection. Moreover, the thick and avascular cortex of the lamina is supposed to prevent intravascular migration of the cement. As stated in the “Materials and Methods” section, we performed all the procedures under fluoroscopic guidance; but at the operator's preference, CT fluoroscopy might be of assistance during precise needle placement in the spinous processes and during PMMA injection. However, traditional fluoroscopy would still be necessary for the interspinous spacer-device placement.
One additional concern regarding spinoplasty is the surgical difficulties that could be encountered, due to intraosseous presence of cement, in those patients affected by INC who have interspinous spacer placement and fail to improve, thus needing to undergo decompressive laminectomy. The recommended cement filling of only the medial portions of the laminae maintains free (not interested by cement injection) the usual point of surgical resection of the laminae. Nevertheless, the balance point regarding this possible issue most likely stands in the appropriate selection of patients, with regard to a degree of canal and/or foraminal stenosis expected to be treatable by interspinous spacer implant, therefore allowing avoidance of a subsequent escalation to a surgical measure. Conversely, risk factors for bone fragility, such as age older than 75 years, make most of these patients poor surgical candidates.
Conclusions
Posterior spinoplasty in patients undergoing interspinous spacer device placement has a biomechanical rationale, is easy and minimally invasive, requires only local anesthesia, and is relatively inexpensive. Our data suggest that this technique is safe in elderly or fragile patients and is effective in preventing delayed fracture of the spinous processes. Given the large effect we observed in this clinical trial along with the absence of important drawbacks in the study design, we decided to continue using this technique on our future patients. However, considering the small sample size and the absence of randomization, patients will be strictly followed up to monitor the results. Unless this monitoring raises doubts on the efficacy of this technique, we will continue to offer posterior arch cement injection to patients at risk of bone fragility in our center. Longer follow-up and confirmations of our data through randomized trials performed external to our group are warranted.
Acknowledgments
We thank Quintella Grant and Lucas Sheldon, MDF, for assistance during the manuscript editing
Footnotes
-
Paper previously presented at: Annual Meeting of the American Society of Spine Radiology, July 1–2, 2011; Honolulu, Hawaii. It was awarded the prize as best scientific paper in the Interventional Section.
-
Disclosures: Alessandro Marrocu—UNRELATED: Employment: 36000, Comments: Research and Development Engineer for 2B1 S.r.l. Medical Device.
References
- Received April 16, 2011.
- Accepted after revision June 15, 2011.
- © 2012 by American Journal of Neuroradiology