Abstract
BACKGROUND AND PURPOSE: Vertebral augmentation is an established treatment for painful osteoporotic vertebral fractures of the spine. Nevertheless, patients may continue to have significant back pain afterward. The purpose of this study was to assess the source of persistent or recurrent back pain following vertebral augmentation.
MATERIALS AND METHODS: Our institutional review board approved this study. We evaluated 124 consecutive patients who underwent vertebral augmentation for painful osteoporotic vertebral fractures. All patients were evaluated after 3 weeks, 3 months, and 1 year following their procedure. Patients with any type of back pain after their procedure were examined under fluoroscopy.
RESULTS: Thirty-four of 124 (27%) patients were men, and 90/124 (73%) were women. Persistent or recurrent back pain, not due to a new fracture or a failed procedure, was present in 29/124 (23%) patients. The source of pain was most often attributed to the sacroiliac and/or lumbar facet joints (25/29 or 86%). Seventeen of 29 (59%) patients experienced immediate relief after facet joint injection of a mixture of steroid and local anesthetic agents. The remaining 12 (41%) had relief after additional injections. Ten (34%) patients ultimately required radio-frequency neurolysis for long-term relief.
CONCLUSIONS: Back pain after vertebral augmentation may not be due to a failed procedure but rather to an old or a new pain generator, such as an irritated sacroiliac or lumbar facet joint. This is of importance not only for further pain management of these patients but also for designing trials to compare the efficacy of vertebral augmentation to other treatments.
ABBREVIATION:
- SIJ
- sacroiliac joint
Vertebral compression fractures are the most common osteoporosis-related injury, with an estimated incidence of approximately 700 000 cases per year in the United States.1 These fractures can cause considerable pain, disability, and morbidity with minimal or slow improvement of the pain with conservative nonsurgical treatment, which leads to substantial decline in quality of life.2–6 Percutaneous vertebral augmentation is an established treatment for painful vertebral compression fractures of the spinal column with rapid pain relief, improvement in mobility, and decreased disability and cost effectiveness.7–11 Vertebral augmentation is a broad term that includes vertebroplasty and kyphoplasty. These are percutaneous image-guided invasive procedures for the treatment of painful vertebral compression fractures, in which acrylic bone cement is injected into the damaged vertebral body.
Numerous case series and 2 randomized controlled trials comparing these augmentation procedures with conservative management have shown significant beneficial effects in favor of vertebral augmentation.8,11–13 Unfortunately, some patients may continue to experience substantial back pain after their vertebral augmentation procedure. In those cases in which there is no incident vertebral compression fracture, this may not necessarily reflect a lack of efficacy of the vertebral augmentation procedure, as suggested by 2 recent studies, or a failed procedure, in which there is persistent vertebrogenic pain at the treated vertebral level, but rather pain may be due to an old or a new pain generator such as an irritated sacroiliac, thoracic, or lumbar facet joint.14,15 The anatomic changes associated with the fracture deformity may adversely affect the facet or SIJs. These irritated or damaged structures are unlikely to be affected by the vertebral augmentation procedures but might demonstrate a favorable response to the injection of a local anesthetic agent.16 The purpose of this study was to assess the source of persistent or recurrent thoracic or lower back pain following a vertebral augmentation procedure and to assess the efficacy of subsequent treatments.
Materials and Methods
Patient Selection
Our institutional review board approved the study, and Health Insurance Portability and Accountability Act—compliant practices were used throughout our investigation. We evaluated 124 consecutive patients who underwent vertebral augmentation for painful osteoporotic vertebral compression fractures at our institution retrospectively during a 2-year period.
All of these patients were referred for a vertebral compression fracture consultation at our institution after a thorough clinical evaluation, including either MR imaging or skeletal scintigraphy and CT of the spine, by their clinicians, and demonstrated the presence of ≥1 osteoporotic vertebral compression fracture as the possible cause of their back pain symptoms. Most of these vertebral compression fractures were subacute (6–12 weeks after the development of back pain symptoms), and these patients were not responding to conservative management with bed rest, analgesics, or orthosis. After an additional detailed clinical evaluation and fluoroscopic examination at the time of consultation, these patients were determined to be candidates for vertebral augmentation. All patients undergoing vertebral augmentation were subsequently evaluated after their procedures at standard time intervals (3 weeks postprocedure, 3 months postprocedure, and 1 year postprocedure) to assess patient outcome and provide additional patient management. In those patients with recurrent or persistent post–vertebral augmentation pain, the source of pain and the efficacy of subsequent spine-injection treatments were assessed.
Vertebral Augmentation Technique
The vertebral augmentation procedures were performed in an interventional radiology suite by using a biplane fluoroscopy unit (Integris V5000; Philips Medical Systems, Eindhoven, the Netherlands) with pulsed fluoroscopy at a rate of 7.5 pulses/second. The procedures were performed by an experienced interventional neuroradiologist under strict aseptic technique and local 2% anesthesia at the puncture site, with administration of the local anesthetic agent to the level of the periosteal surface. All patients were sedated with either intravenous propofol or midazolam (Versed) and fentanyl and were monitored by an anesthesiologist. Patients underwent either kyphoplasty or vertebroplasty by using bilateral or unilateral approaches with transpedicular or parapedicular access to treat vertebral compression fractures. Vertebroplasty was performed in patients whose vertebral body fractures showed only mild height loss, in patients with upper thoracic (T1 through T6) vertebral compression fractures, and in situations in which extensive patient comorbidities allowed only mild sedation to be administered. We used a 10-cm-long 10.5-ga bone needle for the vertebroplasty procedure and coaxial cannulas for cement injection.
Kyphoplasty was performed in patients whose vertebral compression fractures demonstrated at least 20% height loss, vertebral compression deformities with near-complete height loss (vertebra plana configuration), and vertebral compression deformities with significant avascular necrosis and endplate deformities or defects. Either 8- or 10-ga bone-access needles were used for kyphoplasty, depending on the size of the vertebral pedicle. Inflatable balloon tamps were used for the purpose of cavity creation and vertebral endplate remodeling in all of the patients undergoing kyphoplasty. Cement injection with commercially available polymethylmethacrylate bone cement (CONCERT Spine VR, Advanced Biomaterial Systems, Chatham, New Jersey) was performed by a hand-injection technique with a plunger by using coaxial bone-filler cannulas with both vertebral augmentation techniques by using detailed fluoroscopic monitoring. End points for termination of cement injection included satisfactory filling of the anterior two-thirds of the vertebral body or satisfactory cement filling of a large cleft or cement beginning to encroach upon the disk-endplate interface or basivertebral plexus. Cement volumes averaged 2.3 mL in the thoracic spine and 3.5 mL in the lumbar spine. Intraoperative-procedure time averaged approximately 20 minutes per level treated. The skin incision site was secured with Steri-Strip bandages (Nexcare 3M, Maplewood, Minnesota). All patients were monitored and recovered for 3 hours after their procedure. Most of these procedures were performed on an outpatient basis, with inpatient procedures performed in patients in whom a vertebral compression fracture was subsequently identified as the source of their abdominal or chest pain, in patients with intractable back pain, or in patients requiring transient correction of their anticoagulation status.
Pain Management
All patients were routinely evaluated and examined in the clinic at 3 weeks, 3 months, and 1 year after their vertebral augmentation procedure by the same operator who performed the initial vertebral augmentation procedure and by a nurse practitioner. This evaluation included a medical history, pain diagram with a numeric pain scale from 0 to 10, and a physical examination. Patient history was obtained by the nurse practitioner, and the pain diagram and numeric pain score was reported and provided by each patient. Patients with any type of back pain after the procedure were examined under fluoroscopy, by the same operator who performed their initial vertebral augmentation procedure, to better assess the postoperative site, assess the presence of a new vertebral compression fracture, or identify the site of pain along the spinal axis. Patients with vertebral body-related or vertebrogenic pain and a suspected vertebral compression fracture were referred for MR imaging to assess the presence of a new vertebral compression fracture. Those patients with a vertebrogenic pain source, focal palpable midline spinal tenderness, and a new vertebral compression deformity were considered to have a new vertebral compression fracture.
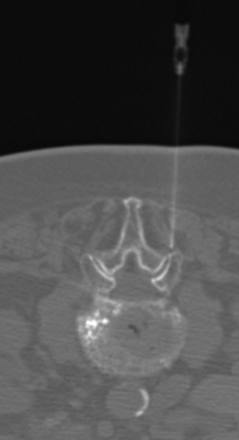
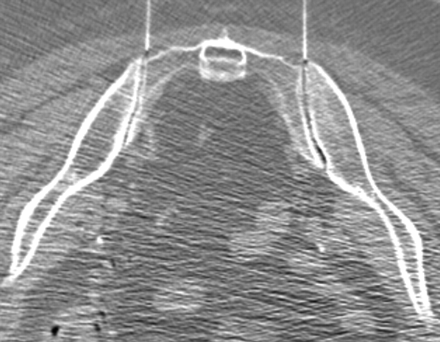
Patients with facet joint- or SIJ-related pain had pain diagrams that lateralized the side of pain to the affected joint or joints. These patients had focal palpable tenderness over the affected joint or joints. Those patients in whom the facet joint or SIJ was identified as a potential pain generator were subsequently scheduled for therapeutic injection of the affected joint (Figs 1 and 2). These procedures were performed on an outpatient basis by an experienced interventional neuroradiologist using either fluoroscopic or CT fluoroscopic guidance. A 22-ga spinal needle was advanced into the affected joint under imaging guidance. Arthrography was then performed with 1–3 mL of iodinated low-osmolar contrast media to confirm the intra-articular location of the needle tip. The joint was subsequently injected with 1–3 mL of a mixture of 2 mL (80 mg) of methylprednisolone and 3 mL of 0.5% bupivacaine. This volume of injectant allowed both intra- and periarticular distribution of the medication in all patients who were treated. The patient was questioned regarding pain provocation at the time of joint injection, and the response was documented. All patients were monitored and recovered for 30 minutes before discharge.
CT-guided unilateral facet joint injection. The patient is a 97-year-old woman (patient 19 in Table 1) with recurrent back pain after T10 and T11 vertebral augmentation. Her initial back pain symptoms were localized to the right upper lumbar facet joints under fluoroscopic evaluation. She was pain-free after undergoing right L1-L2 and L2-L3 facet joint injections 2 weeks after vertebral augmentation. She returned 11 months after her vertebral augmentation procedure for new low-back-pain symptoms that localized to her SIJs and responded to bilateral SIJ injections.
CT-guided bilateral SIJ injection. This 74-year-old woman (patient 28 in Table 1) had recurrent low back pain after T12 vertebral augmentation. On evaluation, including a pain diagram and fluoroscopic examination, her pain localized to her SIJs. Her T12 level revealed no evidence of palpable tenderness, and there was no new vertebral compression fracture. She became pain-free after 2 sessions of bilateral SIJ injection 6 weeks and 7.5 months after T12 vertebral augmentation.
Radio-frequency neurolysis was performed in patients who showed a favorable but short-term response, 2–4 weeks, to facet or SIJ injection.
In 8 patients with thoracic or lumbar facet-related pain, diagnostic median branch blocks were required before proceeding with radio-frequency neurolysis. Sacral radio-frequency neurolysis was performed in 2 patients who experienced temporary pain relief following their SIJ injections. These 2 patients showed a favorable pain response to diagnostic anesthetic injections in their SIJs before undergoing sacral radio-frequency neurolysis with a bipolar technique and treatment of the dorsal branches of S1, S2, and S3 as well as monopolar treatment at L5.
All 29 patients were seen in follow-up to assess their response to treatment. The patients were again required to complete a pain diagram and a numeric pain scale at the time of their evaluation. All patients were referred for outpatient physical therapy at facilities close to their homes, either after their 3-week evaluation or after their spine-injection procedures. Physical therapy was directed at spine rehabilitation, with core muscle strengthening, gait, and balance training. Additionally, patients with SIJ pain underwent pelvic and sacroiliac stabilization exercises and maneuvers as part of their physical therapy regimen.
Statistics
Because some patients underwent multiple vertebral augmentation procedures and multiple joint injections, we decided to use the averages of each vertebral body treatment and joint injection treatment to compare the level of joint injection with vertebral augmentation levels. Each vertebra was assigned a number starting from 1 for C1 to 24 for L5 and 25 for any sacral region (S1-S5 or SIJ). The average of the locations augmented was compared with the average of the locations that were treated for post–vertebral augmentation pain by using a paired t test, and the difference and confidence interval for the difference were reported. A P value < .05 was considered significant. The Mann-Whitney U test was used for any unpaired comparison without normal distribution. The normality of the distribution was assessed by the D'Agostino-Pearson omnibus test (GraphPad Prism, Version 5.02 for Windows; GraphPad Software, San Diego California; www.graphpad.com).
Results
In this study, we evaluated 124 consecutive patients who underwent vertebral augmentation procedures. The average age of the patients was 79 ± 11.6 years. We had 34 (27%) male and 90 (73%) female patients. One procedure-related complication occurred in this series of patients. A patient developed diskitis and osteomyelitis at the level of treatment. This condition responded to antibiotic therapy. Vertebroplasty and kyphoplasty were performed on 65 (25%) and 190 (75%) levels, respectively, with a total of 255 levels (mean, 2.1 levels in each patient) treated. A transpedicular approach was used for 211 (83%) levels, and a parapedicular approach was used in 44 (17%) levels. One hundred ninety-five (76%) levels were treated unilaterally, and 60 (24%) levels were treated by a bilateral approach.
The immediate preprocedural pain scores ranged from 7/10 to 10/10 in this group of 124 patients. The postprocedure pain scores after vertebral augmentation ranged for 0–3 of 10 in this patient cohort. Forty-two of the 124 patients (34%) presented with persistent or recurrent back pain during their follow-up clinical evaluations. Seventeen (14%) of the 124 patients had pain due to a new thoracic or lumbar vertebral compression fracture as seen on fluoroscopic evaluation, with palpable midline spinal tenderness corresponding to the injured vertebra and clinical examination, or on the MR imaging study in those patients with a suspected vertebral compression fracture. Fourteen of these 17 patients with new fractures underwent additional vertebral augmentation procedures with subsequent pain relief in 10 and persistent or recurrent back pain in 4 patients. The remaining 3 patients were managed conservatively as per their clinician's discretion due to the presence of other comorbidities.
None of the patients in this series demonstrated new radicular symptoms or clinical findings to suggest radiculopathy at the time of their clinical evaluations. A total of 29 (23%) of the 124 patients, including the above-mentioned 4 patients, had persistent or recurrent back pain after vertebral augmentation, which was not due to a new fracture or a failed procedure (Table 1). “Persistent pain” is defined as the patient's continuous experience of pain after the vertebral augmentation period, regardless of severity, without any significant periods, according to the patient, of pain relief. “Recurrent pain” is defined as new back pain that occurs after a pain-free period following the vertebral augmentation procedure; 23 of the 29 patients experienced recurrent back pain. The SIJ was the only source of pain in 15 patients (52%), while the facet joints were the only source of pain in 8 of the 29 patients (27%). Both the SIJs and facet joints were a source of pain in the remaining 6 patients (21%). There was no significant difference between the presence or absence of post–vertebral augmentation back pain with respect to either the number of fracture levels treated, the treatment method (vertebroplasty versus kyphoplasty), or the approach (ie, unilateral versus bilateral or transpedicular versus parapedicular) (Table 2, Mann-Whitney U test).
Demographic data, the regions treated with vertebral augmentation, and the type of post–vertebral augmentation pain management
Comparison of the number of levels treated regardless of procedure type, number of levels treated by kyphoplasty versus vertebroplasty, number of levels treated by transpedicular versus parapedicular approach, and number of levels treated by unilateral versus bilateral approach
To compare the location of a patient's clinically palpable back pain relative to their previously treated vertebral compression fracture, we recorded the levels of treatment for each procedure. Detailed physical and fluoroscopic examinations of the back showed no evidence of palpable midline spinal tenderness at the level of the treated vertebral compression fracture, as was the case before the fracture treatment; in other words, palpable spinal tenderness was always present at the fracture level before vertebral augmentation but not after the vertebral augmentation procedure.
Comparison of the specific vertebral levels treated with vertebral augmentation and the specific joint levels treated with subsequent joint injection and/or radio-frequency neurolysis showed a significant difference (P < .01). On average, the post–vertebral augmentation source of pain treated by joint injection or radio-frequency neurolysis was 3.8 levels (95% CI, 1.4) lower than the vertebral fracture treatment level (P < .01). Seventeen of 29 (59%) patients had complete pain relief after a single session of treatment. Twelve of 29 patients (41%) had pain relief after additional treatments with an average of 3.3 sessions (range, 2–5 sessions).
Twenty-one patients had SIJ injection. Twenty-four SIJ injections were performed (1 of the patients had 3 sessions and another patient had 2 sessions). Fourteen injections were bilateral, 4 were on the left, and 6 were on the right. Radio-frequency neurolysis was used to treat 10 of 29 patients (34.5%), and 5 of 29 patients (17.2%) underwent epidural steroid injections. The indication for epidural steroid injection in the latter group was lumbar stenosis.
Upon the completion of their spine-injection procedures, all patients experienced significant reduction in their back pain symptoms. Patients who presented initially with pain scores of 7–10 of 10 at their initial vertebral fracture presentation reported similar pain intensities related to their nonvertebrogenic pain generators (for example, sacroiliac or facet). Following the additional spine-injection procedures, these patients reported pain scores in the range of 0–2 of 10, with most patients (25/29, P < .01) reporting no pain.
An additional observation of particular interest was that all patients with recurrent back pain following their initial vertebral augmentation procedure, regardless of their pain generators, thought that their back pain was due to their initial fracture. Many of these patients stated that their vertebral augmentation procedure was not successful because they still were experiencing back pain. A careful review of the pain diagrams in these patients suggested that the pain source could be vertebrogenic in those patients who experienced a subsequent vertebral compression fracture or that the pain source might be joint-related (facet or sacroiliac) in patients with nonvertebrogenic pain profiles. The fluoroscopic examination was extremely helpful in distinguishing these 2 patient groups and in guiding further therapy.
Discussion
This study evaluated the incidence of persistent or recurrent back pain, its source, and the efficacy of joint injection (facet or sacroiliac) in patients treated with vertebral augmentation procedures for osteoporotic vertebral compression. The results demonstrated a 23% (95% CI, 16.4%–31.6%) incidence of significant persistent or recurrent pain causing functional disability and decreased quality of life, not due to a new vertebral compression fracture. This finding was similar to that in another study that showed that 23.6% of patients continued to have residual pain after vertebral augmentation.17 Despite similar incidences, however, most of our patients underwent joint injections while in the aforementioned study; of 34 patients, most of them (26 patients) had epidural steroid injection, 6 had SIJ injection, and only 1 underwent a facet joint injection. The difference in the results may be attributable to the lack of systematic evaluation to identify the source of pain generators responsible for the residual pain in that study, unlike ours. In our study, precision diagnostic techniques using controlled diagnostic blocks were applied. Our study suggests that the facet joints or SIJs are a possible source of residual or new pain in patients who had vertebral augmentation due to osteoporotic fractures.
Our results showed significant pain relief in all of those patients with persistent or recurrent pain not due to a new fracture after single or multiple joint injections. Review of the literature showed different results in pain relief after facet injection.18–20 A randomized double-blind controlled trial with a 2-year follow-up showed a very high rate of significant pain relief in 85%–90% of patients with chronic back pain after lumbar facet injection.18 However, another systematic review by Boswell et al19 showed that the evidence for short- and long-term pain relief is moderate for lumbar facet joint injection. Another systematic review of randomized controlled trials by Staal et al20 showed that there is insufficient evidence for or against facet joint injection and that specific subgroups of patients may benefit from treatment.20 The above-mentioned studies assessed the efficacy of facet joint injection in participants who had subacute or chronic back pain with various causes, and the results may not be applicable to our patients.18–20 To the best of our knowledge, there is no study available in the literature that evaluates the efficacy of facet and/or SIJ injection in patients with persistent or recurrent back pain after vertebral augmentation.
Our results showed no significant difference in the number of levels treated, treatment type (kyphoplasty versus vertebroplasty), and the technique (number of levels treated by transpedicular versus parapedicular approach, and number of levels treated by unilateral versus bilateral approach) in patients with persistent or recurrent pain compared with the patients without residual pain. Although randomized controlled clinical trials with several years of follow-up are required to compare vertebroplasty, kyphoplasty, and the differences in techniques to provide definitive data for optimal treatment of osteoporotic vertebral compression fractures, our results are consistent with those in the literature that show that there is no significant difference in pain relief regardless of the procedure type and the technique used.21–25
A limitation inherent in all retrospective studies of this nature is the lack of a control group to compare the above-mentioned interventional techniques (ie, SIJ and facet joint injections, epidural injections, and radio-frequency neurolysis) with a cohort who only had vertebral augmentation. Additionally, many patients could not be effectively evaluated initially for facet or SIJ pain because they had severe dominant vertebrogenic pain. The prevertebral augmentation pain diagrams, however, did not suggest that there was a potential SIJ pain generator in those patients who subsequently developed SIJ-related pain. Unfortunately, it is not always possible to discern which patient already had joint pain, which patient sustained joint injury at the moment of their fracture event, and which patient developed joint pain afterward. In the latter case, it has been demonstrated in the literature that invasive spine procedures, such as fusion with or without instrumentation, can predispose patients to facet joint or SIJ pain.26 Last, it has been shown that patients with vertebral compression fractures who do receive perifacet injections do achieve at least transient pain relief.14
Conclusions
Back pain after vertebral augmentation is not necessarily due to a failed procedure, but rather it could be due to an old or a new pain generator such as an irritated SIJ or lumbar facet joint. This is of importance in further management of these patients as well as for designing trials to compare the efficacy of vertebral augmentation with that in other treatments.
Footnotes
-
Disclosures: Orlando Ortiz—UNRELATED: Consultancy: Orthovita, Comments: Speakers' Bureau (1 conference/year); Payment for Lectures, Including Service on Speakers Bureaus: Medtronic Spine, Comments: 1-hour lecture given at American Society of Spine Radiology Symposium.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received April 5, 2011.
- Accepted after revision June 7, 2011.
- © 2012 by American Journal of Neuroradiology