Abstract
BACKGROUND AND PURPOSE: DAVFs with cortical venous reflux carry a high risk of morbidity and mortality. Endovascular treatment options include transarterial embolization with a liquid embolic agent or transvenous access with occlusion of the involved venous segment, which may prove difficult if the venous access route is thrombosed. The aim of this article is to describe the technique and results of the transvenous approach via thrombosed venous segments for occlusion of DAVFs.
MATERIALS AND METHODS: Our study was a retrospective analysis of 51 patients treated with a transvenous approach through an occluded sinus that was reopened by gentle rotational advancement of a 0.035-inch guidewire, which opened a path for a subsequently inserted microcatheter.
RESULTS: Of 607 patients with DAVFs, the transvenous reopening technique was attempted in 62 patients in 65 sessions and was successful in 51 patients and 53 sessions. Immediate occlusion was seen in 42 patients; on follow-up, occlusion was seen in 49 patients, whereas 2 patients had reduced flow without cortical venous reflux. No permanent procedure-related morbidity was noted.
CONCLUSIONS: The reopening technique to gain access to isolated venous pouches or the cavernous sinus for the treatment of DAVFs is a safe and effective treatment, which should be considered if transarterial approaches fail or are anticipated to result only in an incomplete anatomic cure.
Abbreviations
- AP
- anteroposterior
- AV
- arteriovenous
- AVF
- arteriovenous fistula
- ECA
- external carotid artery
- DAVF
- dural arteriovenous fistula
- DSA
- digital subtraction angiography
- ICA
- internal carotid artery
- IPS
- inferior petrosal sinus
- MRA
- MR angiography
- n-BCA
- n-butyl 2-cyanoacrylate
- SSS
- superior sagittal sinus
DAVFs are defined as abnormal communications between the venous sinus system and dural branches of the internal carotid, external carotid, and vertebral arteries and rarely by cortical vessels. While their exact etiology is not known, acquired lesions and increased angiogenetic activity are presumed to occur following various triggering factors (such as venous thrombosis, trauma, or previous surgery), which have been put forward as potential causes for these lesions.1 Clinical presentations vary depending on the location of the shunt, the presence or absence of cortical venous reflux, and the shunt volume.2 DAVFs with cortical venous reflux carry a high risk for neurologic sequelae or death,3 and presentation with hemorrhage is considered a risk factor for future hemorrhage.4–6
There are different classifications for DAVFs, including the Borden7 and Cognard8 classifications, which are based on the cortical venous reflux pattern and are, therefore, helpful in predicting natural history. A recent classification from the Bicetre group focused on the anatomy of the cranial epidural spaces, which may help in subdividing different types of dural AV shunts.9,10 Therapeutic strategy in most centers is based on the presentation of the patient and the pattern of venous drainage, and it is generally accepted that lesions with cortical reflux have to be treated with the aim of complete obliteration of the reflux into the cortical veins.11 Treatment options are manifold and include endovascular treatment (both via a transarterial approach or a transvenous approach), surgery,12 radiosurgery,13,14 or conservative treatment (eg, manual eye compression for low-flow cavernous sinus dural fistulas without reflux15). For all treatment techniques, the goal must be to occlude the fistulous zone; a proximal arterial ligation will never be sufficient.
Recent studies have shown very good outcome with low complication rates for occluding DAVFs via a transarterial route by using liquid embolic agents16; however, this route may not always be feasible, especially if multiple small feeders are present and shunt into a widely dispersed segment of the dural wall. In these instances, venous approaches may be considered.17–22 In patients with “trapped sinuses” and in certain anatomic dispositions, a transvenous access is not readily feasible. Therefore, different groups have reported combined surgical and endovascular approaches, including surgical superior ophthalmic venous access or burr-hole craniotomy with subsequent endovascular treatment or purely surgical approaches with sinus packing or surgical excision.23–31 However, in addition to these surgical techniques, a few groups have described entering a trapped sinus via the occluded venous segment using a purely endovascular technique.29,32,33 The aim of this article is to describe, in the largest series of patients so far from 2 different hospitals, the venous reopening technique to treat dural AV shunts via a transvenous approach through an occluded segment, including angiographic and clinical outcome and complication rates.
Materials and Methods
Following institutional ethics review board approval, a retrospective analysis of the dedicated neurovascular data banks of the 2 participating hospitals was conducted, which identified all patients in whom transvenous treatment via an occluded venous segment with the technique mentioned below was performed. The data bases consisted of 374 (Toronto Western Hospital) and 233 (Ramathibodi Hospital) intracranial DAVFs, respectively, with patients presenting between January 1998 and June 2010. Both data bases had been collected prospectively since 1989 by a team of neuroradiologists and neurosurgeons in a multidisciplinary clinic. After identification of patients in whom the transvenous reopening technique was either performed or attempted, age, sex, clinical presentation, angiographic findings, treatment techniques, angiographic/clinical outcomes, and complications of the reopening technique for DAVFs were retrospectively reviewed. In all patients, bilateral selective ICA, ECA, and vertebral artery angiographies were available and were assessed for feeding arteries, locations, and venous drainage patterns of the DAVFs, which were reviewed and classified by an experienced neurointerventionalist according to the Borden, Cognard, and Bicetre classification schemes. The inclusion criterion for this retrospective review was a DAVF treated transvenously via an occluded venous segment (ie, via a nonopacified venous segment). The patients were followed up clinically on the day after the procedure and 1–3 months after treatment and were assessed for any new neurologic deficits. MR imaging, including time-resolved MRA or DSA, was performed to verify occlusion of the fistula.
Description of the Technique
Arterial and venous access is gained via a femoral approach with the patient under general anesthesia. Following diagnostic arterial angiography, a 5F diagnostic catheter with a continuous flush is placed in the vessel that best demonstrates the shunt and the target structure (ie, the trapped sinus). A 6F guiding catheter is advanced transvenously via the internal jugular vein and placed at the jugular bulb (for those lesions located at the cavernous sinus or transverse sigmoid sinus), at the sigmoid sinus (for lesions located at the torcular region), or at the transverse sinus (for lesions located at the SSS). Transverse and sigmoid sinus locations may require a more stable access with a shuttle sheath through which the guiding cathether is advanced. Via the 6F guiding catheter, a 0.035- or 0.038-inch hydrophilic guidewire is gently advanced with continuous rotation under roadmap (phlebogram) from the arterial injection toward the occluded sinus. For DAVFs located at the cavernous sinus, the guidewire is inserted into the medially and superiorly pointing IPS, with the unsubtracted fluoroscopy or the arterial roadmap as an anatomic guidance (Fig 1 B, -C). The guiding catheter is gently followed into the proximal IPS for increased stability. The guidewire is further advanced superiorly toward the right cavernous sinus, thus creating a tract for a microcatheter. For DAVFs located at the transverse sigmoid sinus and the SSS, the guidewire is pointed laterally toward the occluded sigmoid sinus again by using anatomic landmarks from the fluoroscopic image as guidance (Fig 2 D), to reopen a small tract through the occluded sigmoid sinus and SSS (Fig 3), respectively.
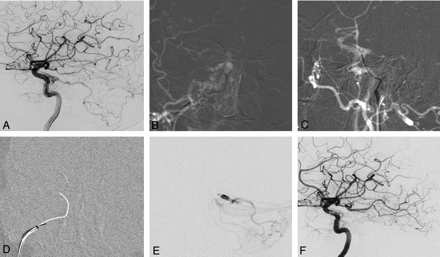
DAVF of the cavernous sinus with reflux into the posterior fossa. A 76-year-old woman presented with right-sided chemosis and diplopia. A, Right internal carotid angiogram shows a DAVF at the cavernous sinus fed by cavernous branches and the meningeohypophyseal artery of the ICA with marked posterior fossa reflux. There is no flow visible into the IPS. B and C, Following an arterial roadmap, AP view (B) and lateral view (C), the guiding catheter is oriented superiorly, medially, and anteriority toward the presumed origin of the IPS. A 00.35-inch guidewire is used to reopen the occluded IPS by gentle rotation and is advanced through the occluded sinus under roadmap guidance. D, Once access is gained with the guidewire as demonstrated by the arterial roadmap, a blank roadmap is initiated, and the guidewire is removed, and a track is left for the microcatheter to enter the cavernous sinus. E, The location of the microcatheter is checked by a careful contrast injection before coil embolization to demonstrate the exact origin of the cortical venous reflux and to verify that the right compartment is coiled. F, Right internal carotid angiogram following embolization demonstrates complete obliteration of the DAVF.
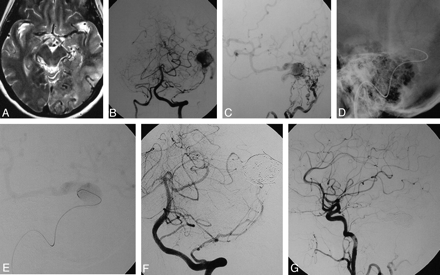
DAVF into a left “isolated” transverse sinus with cortical venous reflux. This 59-year-old female patient presented with alteration of consciousness and vertigo. A, Axial T2-weighted image shows dilation of cortical veins and hypersignal of the brain parenchyma as a sign of venous congestion in the left temporal and occipital lobes. B and C, Vertebral artery injections in an AP plane (B) and lateral occipital artery injections (C) demonstrate a DAVF at the left transverse sinus with an isolated enlarged venous pouch and cortical venous reflux with supply from multiple small branches of the occipital arteries and a dural branch of the left vertebral artery. D, With the guiding catheter in the left jugular bulb, a 0.035-inch guidewire is advanced through the occluded sigmoid sinus into the venous pouch. E, The track is used by a microcatheter, and the position of the microcatheter is checked with a careful injection once in the venous pouch. F and G, Following packing of the sinus with coils, control angiograms of the left vertebral artery (F) and the left common carotid artery (G) demonstrate complete obliterations of the DAVF.
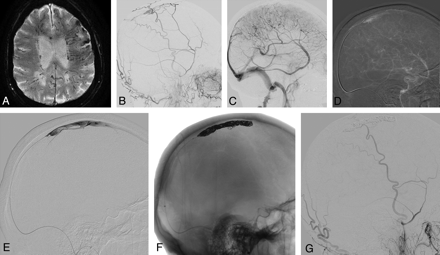
Dural AVF of an isolated SSS with cortical venous reflux. An 87-year-old man presented with seizure, alteration of consciousness, and progressive weakness of his right hand. A, A T2 gradient-echo sequence demonstrates multiple dilated transmedullary veins and cortical microhemorrhages as a sign of chronic venous congestion. B and C, ECA (B) and ICA (C) runs demonstrate a DAVF into the SSS with occlusion at the distal third of the SSS with venous congestion. A 6F Shuttle-SL guide sheath (Cook, Bloomington, Indiana) is placed into the right jugular bulb, and a 6F Neuron (Penumbra, Alameda, California) catheter is advanced into the proximal SSS. D, From here, a 0.035-inch guidewire is advanced into the venous pouch. E–G, Via the thus-opened track, a microcatheter is advanced (E) with confirmation of the tip of the catheter in the SSS, and complete occlusion of the isolated sinus with coils is performed (F), leading to complete obliteration of the DAVF (G).
Several channels leading to different compartments of the cavernous sinus but also the transverse sinus are usually encountered, and multiple attempts may be necessary to find the correct compartment (ie, the compartment into which the shunt occurs). Once the guidewire penetrates into the venous sinus (which is best visualized by the more extensive movement of the guidewire tip when rotating), a blank roadmap is obtained. Before the guidewire is removed, a 2-tip microcatheter is prepared (we recommend an inner lumen of at least 0.021 inches, which allows deployment of highly thrombogenic pushable 18-fibered coils) with a microguidewire (0.016-inch microguidewires are more stable and allow more “pushability” and may, therefore, be recommended over standard 0.014-inch guidewires). Then the 0.038-inch guidewire is withdrawn, leaving a track on the blank roadmap (Fig 1D), which must be followed with the previously prepared microcatheter-microguidewire as quickly as possible because occlusion of the track occurs rather soon following withdrawal of the reopening guidewire. Once the microcatheter is inside the trapped sinus, injection of contrast through the microcatheter is performed to confirm its position (Fig 1E and Fig 2E). Washout of contrast (from the noncontrasted arterial blood still entering the fistula) is seen and enables correct identification of the fistulous zone. Once the microcatheter is in the correct position, transvenous occlusion of the fistulous zone can be performed, taking care not to lose access (ie, being pushed back into the occluded sinus) and to include the most proximal part of all potential cortical veins, which may drain the venous pouch to prevent redirection of the fistulous flow toward the brain. In our practice, we start with detachable coils to perform a frame, followed by multiple fibered platinum coils to induce thrombosis in the venous segment that harbors the shunt. Arterial control angiograms are obtained intermittently to evaluate the type and volume of the residual arteriovenous shunt and its venous drainage. Once closure of the DAVF is obtained, the microcatheter and the venous guiding catheters are removed.
Results
Of 607 patients with DAVFs seen at both institutions, the transvenous reopening technique was attempted in 62 patients in 65 sessions and was successfully performed in 51 patients and 53 sessions. In 1 patient with bilateral cavernous sinus fistulas, 1 IPS could be catheterized, while the other IPS could not. One patient underwent a staged embolization with 3 separate sessions; all 3 were successful. In 11 patients with cavernous sinus DAVFs, neither IPS could be catheterized using the above-mentioned technique, resulting in a technical success rate of 53/65 (82%). In 3 cases, we were unable to engage the microcatheter into the obtained channel after the first guidewire pass and had to pass through the nonvisualized vein again with the 0.038-inch microcatheter guidewire, repeating the procedure. In all 3 patients, we were able, during the second attempt, to enter the trapped venous segment.
In the following, we will focus on the 51 successfully treated patients. These 51 patients underwent transvenous embolization by using the above-mentioned reopening technique with access gained through an occluded sinus. Patients' ages ranged from 27 to 87 years, with an average age of 57 years. There were 35 (70%) women and 16 (30%) men. Locations of the shunt were the cavernous sinus in 41 patients (82%), the transverse sigmoid sinus in 4 patients (8%), the transverse sinus only in 3 patients (6%), the torcular transverse sinus in 1 patient (2%), and the SSS in 2 patients (4%).
Arterial feeders to the DAVFs at the cavernous sinus were the meningohypophyseal artery (33 patients), the artery of the foramen rotundum (17 patients), the accessory meningeal artery (14 patients), the inferolateral trunk (13 patients), the internal maxillary artery (13 patients), the middle meningeal artery (13 patients), the ophthalmic artery (5 patients), the ascending pharyngeal artery (4 patients), the stylomastoid artery (3 patients), and the mandibular artery (2 patients). Concerning the shunts of the jugular, sigmoid, transverse, torcular, and SSS, supplying arteries were the occipital artery (9 patients), the middle meningeal artery (9 patients), dural branches of the vertebral artery (6 patients), the ascending pharyngeal artery (3 patients), the meningohypophyseal trunk (3 patients), the accessory meningeal artery (3 patients), the stylomastoid artery (1 patient), the superficial temporal artery (2 patients), and pial-induced supply from the MCA (1 patient). For the entire group, in 6 patients, 1 feeder was present; in 15 patients, 2; in 13 patients, 3; in 11 patients, 4; and in 6 patients, ≥5 feeders were present.
In this series, the venous drainage was classified as Borden type I (drainage into the dural venous sinuses) and Cognard type IIa (venous drainage into the dural venous sinus with retrograde flow but without cortical venous reflux) in 19 patients (38%) (all of whom had a DAVF of the cavernous sinus with reflux toward the eye), and Borden type II (venous drainage in dural venous sinus with cortical venous reflux) and Cognard Type IIa+b (venous drainage into dural venous sinus with retrograde flow and cortical venous reflux) in 32 patients (62%). In this series, malignant neurologic symptoms (seizures, alterations in consciousness or dementia, hemorrhagic complications, nonhemorrhagic neurologic deficits) were present in 12 patients, all of whom had cortical venous reflux.
In 1 patient, a staged approach with 3 subsequent sessions of transvenous treatment using the reopening technique had to be performed to avoid excessive thrombosis in the cortical veins. In the remaining 50 patients, a single session of transvenous embolization was performed. In most, only fibered coils were used as the embolizing agent (n = 33). In 6 cases, fibered coils and bare detachable coils were used; bare detachable coils and HydroCoils (MicroVention Terumo, Aliso Viejo, California) were used in 4 patients; bare detachable coils alone were used in 2 patients; and in 1 patient, HydroCoils and bare detachable and fibered coils were used. Three patients were treated with fibered coils and transvenous n-BCA (glue) injection at the end of the procedure; 1 patient was treated with fibered coils, bare detachable coils, and n-BCA; and 1 patient was treated with bare detachable coils and n-BCA. The number of coils used varied from 1 to 40.
In most patients (46/51), the transvenous reopening technique was the first treatment; in 4 patients, previous transarterial glue injections had been attempted without success. One patient was treated at an outside institution via a surgical burr- hole and subsequent transvenous packing of coils, which, however, did not result in complete obliteration of cortical venous reflux.
Immediately following treatment, no residual shunt was seen in 42 patients; in 1 patient, there was persistent cortical venous reflux, necessitating surgery to obliterate it (Fig 4). In 4 patients with cavernous sinus fistulas, the shunt flow was significantly reduced and manual compression therapy was initiated; in 3 patients the cortical venous reflux was obliterated but the shunt persisted; and in 1 patient, a residual shunt was left intentionally to prohibit excessive cortical venous thrombosis (as detailed above). On follow-up, 2 shunts remained open but did not demonstrate cortical venous reflux, while in the remaining 7 patients (including the surgical patient), the dural AV shunts were completely occluded. One patient in our series with initial complete occlusion was treated with warfarin (Coumadin) and presented 2 months after embolization with recurring eye symptoms (chemosis); MRA demonstrated reopening of the fistula, which was treated by manual compression, which led to complete obliteration on follow-up after 3 months.
Dural AVF with incomplete occlusion following transvenous therapy. A 66-year-old woman presented with proptosis and chemosis of the right eye with mild diplopia. A and B, Cerebral angiogram reveals a caroticocavernous DAVF at the right cavernous sinus with arterial supply from the meningohypophyseal trunk (A) and meningeal branches from the ECA (B), with cortical venous reflux toward the posterior fossa and nonvisualization of the right IPV. With the previously described transvenous reopening technique, a microcatheter is advanced into the cavernous sinus. C, Microcatheter injections reveal the cortical venous reflux. The position of the microcatheter is deemed sufficient for subsequent coil embolization with the aim of first occluding the potential cortical venous reflux toward the posterior fossa. During deployment of the first coil, the microcatheter was pushed back, and access to the posteriorly directed venous outlet toward the posterior fossa was lost. Because access to this outlet could not be regained immediately, it was erroneously deemed sufficient to perform a dense packing of the cavernous sinus to obliterate the shunt. D, While ECA control injections (used during the coiling procedure to control for obliteration of venous reflux) showed obliteration of the DAVF, the final control injection into the ICA, however, still demonstrated reflux to the posterior fossa; and despite multiple attempts, it was not possible to regain access to the vein responsible for the cortical venous reflux, given the coil mass deposited into the sinus. Therefore, surgical disconnection of the remaining cortical venous reflux was deemed necessary. E, During surgical exposure of the cavernous sinus region, the posteriorly directed vein from the cavernous sinus that was responsible for the residual shunt is detected and the vein is coagulated. F, Follow-up angiogram after surgery demonstrates obliteration of the cortical venous reflux. This case demonstrates that it is always necessary to disconnect the cortical venous reflux before obliterating the venous sinus because packing of the sinus may preclude subsequent catheterization of cortical venous outflow tracks. This case also demonstrates that if cortical venous reflux is demonstrated to arise from multiple vessels (in this case both the ICA and the ECA), arterial control injections should be performed in both vessels to verify obliteration of the cortical venous reflux.
There were 5 minor complications and no major complications. One venous perforation of the superior ophthalmic vein occurred in a patient with a cavernous sinus fistula, resulting in ecchymosis, which did not require any treatment and spontaneously resolved. There were no deficits in the patient's vision. In 1 patient, follow-up imaging demonstrated a newly developed subdural hematoma with a maximum width of 5 mm without mass effect and without neurologic symptoms. On follow-up the subdural hematoma vanished. In 1 patient with a high-flow torcular DAVF that shunted into the deep venous system, a small coil dislodged and migrated to the vein of Galen, though it did not lead to thrombosis of the deep venous system. In 1 patient, an arterial dissection of the accessory meningeal artery occurred, which did not lead to neurologic deficits. One patient experienced a painful groin hematoma, which did not require surgery or blood transfusions. There were no permanent neurologic deficits in any patient.
Discussion
DAVFs of the brain can be treated by a variety of techniques, including endovascular routes (both transarterial and transvenous), surgery, radiosurgery, or conservative approaches, including manual compression of low-flow fistulas. In most centers, the primary choice of treatment is via an endovascular approach. The transarterial route aims at occluding the most distal segment of the arterial feeder together with the most proximal part of the vein, which can be accomplished by liquid embolic agents that have a high cure rate with low morbidity and mortality rates. Depending on the angioarchitecture of the shunt and the number and location of the feeding vessels, transarterial routes may, however, not be indicated; instead, a transvenous approach may become necessary. Here the aim will be to exclude the venous segment receiving the arterial input while paying close attention to all potential cortical venous reflux possibilities. The transvenous route is safe if the brain does not use the excluded venous structure for its normal brain drainage. This is always the case if a sinus is trapped (ie, occluded both proximally and distally), and it is possible in nearly all patients with DAVFs of the cavernous sinus, given the potential collateral pathways.
The technique of reopening an occluded transverse sigmoid sinus to gain access to the fistulous zone was first described in 1993 by Gobin et al,32 who treated 2 patients with this technique, resulting in a cure in 1 patient and a significant reduction of flow in the other. Since then, to our knowledge, only a few smaller case series using this technique have been published as detailed below. While our experiences reconfirm those of previous reports as discussed subsequently, we think that the larger number of consecutive patients described herein may give a better estimate of the success and complications rates. In the supplemental On-line Figs 1-8, we additionally describe operative nuances of the technique that may be considered helpful to the neurointerventionalist.
Naito et al29 described 6 patients in whom the reopening technique was attempted. Access was successful in 5 patients, and embolization resulted in a cure in 4. In 1 patient, the sinus was not accessible by using the reopening technique; and in 1 patient, the fistula did not disappear because embolization was performed in a different channel, highlighting the fact that careful anatomic studies have to be performed to determine where the transvenous occlusion will be performed.
Variations of the technique described by Gobin et al32 and Naito et al,29 which was used in our series, were reported by Hanaoka et al,34 who used a snare to pull a microcatheter up into the occluded sinus in 3 patients, and by Halbach et al35 and Komiyama et al,27 who gained access to the isolated sinus from the contralateral side. The latter approach may necessitate the use of a triaxial system to provide better support. A triaxial system is also highly recommended in cases of an isolated SSS DAVF to support the guidewire and the microcatheter when advancing both through the occluded segment. While Sugiu et al36 used a 6F guiding catheter with a 4F catheter and a microcatheter in 2 patients, the setup used by us in those cases necessitating distal catheterization included a 6F shuttle sheath and a 6F flexible guiding catheter (eg, Neuron, Penumbra, Alameda, California; or Fargo Max, Balt, Montmorency, France) (Fig 3). The necessity of having good support was also highlighted by 2 cases reported by Wong et al,31 who attributed their success to the support given by a 6F guiding catheter placed immediately proximal to the occluded sinus.
The proposed approach is less invasive compared with a surgical or combined surgical/endovascular approach (such as gaining access to the isolated sinus via a burr-hole craniotomy with subsequent direct puncture and packing of the sinus as described in 8 patients by Endo et al37) and may, therefore, be the preferred route. Another advantage of the reopening technique is that preoperative flow reduction with particles or glue is not necessary, whereas it may have to be used in surgical cases to avoid significant blood loss.
Access to the cavernous sinus through an occluded or nonopacified IPS was first described by Halbach in 1988 in 2 patients33 and was subsequently used by various groups who reported smaller case series as detailed below. Nonopacification of the IPS when demonstrating the fistula may be due to a variety of reasons. If the fistula uses alternate drainage pathways due to compartmentalization of the cavernous sinus, the IPS may be visualized in the later venous phases (as a potential drainage pattern of the normal brain). If the IPS is visualized on neither early nor late phases, it may be anatomically absent or thrombosed. In the latter cases, a phlebogram of the jugular bulb is helpful because it may demonstrate the proximal remnant of the IPS as described by Benndorf et al.25 In the series of Shiu et al,38 the IPS could not be catheterized in 31% of cases due to its plexiform architecture. This corresponds well to our series. Of the 62 attempted patients, 52 had lesions in the cavernous sinus, of which we were able to treat 41, whereas in the remaining 11 patients with cavernous sinus DAVFs (27%), access could not be gained. The approach to the cavernous sinus via the IPS is considered safe, even in cases in which the IPS is not visualized. Benndorf et al25 had no complications in their series of 14 patients and reviewed the literature concerning reported complications. They found 6 patients, 4 of whom could be managed via an endovascular approach with no remaining deficits.
Complications may occur due to the increase of intravenous pressure during contrast injections, which can be avoided by careful test injections to verify the catheter position before performing more powerful microcatheter injections.39 However, perforation may also occur due to advancement of a sharp wire tip, stiffened by the catheter and the narrow vascular structure.39 This complication may be avoided when using the more rounded (and therefore less traumatic) 0.035-inch guidewire under constant gentle rotation with very slow advancing motions. The authors think that a microguidewire, especially when stiffened by the microcatheter and when just barely advanced over the tip of the microcatheter, carries a higher risk of complications because it may be more traumatic being rather sharp at its tip, whereas the tip of a 0.035-inch guidewire is rounded and, therefore, less traumatic, especially when it is constantly rotated. The use of a loop or a J-configuration of the microguidewire may, however, constitute an alternative to the 0.035-inch guidewire approach.25 Once access is gained to the cavernous sinus, careful anatomic evaluation of all potential venous outflow tracts is mandatory to avoid rerouting of the arterialized blood toward the brain. A very dense coil mesh carries the risk of cranial nerve palsy due to mass effect, which is why we recommend thrombogenic fibered coils rather than bare platinum or HydroCoils.
Conclusions
The reopening technique to gain access to isolated venous pouches or the cavernous sinus for the treatment of DAVFs is a safe and effective treatment, which should be considered if transarterial approaches fail or are anticipated to result only in incomplete anatomic cure. The main points to consider in this treatment are a stable guiding catheter position; gentle rotational advancement of a 0.035-guidewire, which creates a track for the subsequently advanced microcatheter; creation of a roadmap by pulling out the wire; and careful analysis of all potential venous outflow tracts to avoid rerouting of flow toward the brain.
References
- Received November 8, 2010.
- Accepted after revision January 6, 2011.
- © 2011 by American Journal of Neuroradiology