Abstract
BACKGROUND AND PURPOSE: The regional leptomeningeal score is a strong and reliable imaging predictor of good clinical outcomes in acute anterior circulation ischemic strokes and can therefore be used for imaging based patient selection. Efforts to determine biological determinants of collateral status are needed if techniques to alter collateral behavior and extend time windows are to succeed.
MATERIALS AND METHODS: This was a retrospective Institutional Review Board–approved study of patients with acute ischemic stroke and M1 middle cerebral artery+/− intracranial internal carotid artery occlusion at our center from 2003 to 2009. The rLMC score is based on scoring pial and lenticulostriate arteries (0, no; 1, less; 2, equal or more prominent compared with matching region in opposite hemisphere) in 6 ASPECTS regions (M1–6) plus anterior cerebral artery region and basal ganglia. Pial arteries in the Sylvian sulcus are scored 0, 2, or 4. Good clinical outcome was defined as mRS ≤2 at 90 days.
RESULTS: The analysis included 138 patients: 37.6% had a good (17–20), 40.5% a medium (11–16), and 21.7% a poor (0–10) rLMC score. Interrater reliability was high, with an intraclass correlation coefficient of 0.87 (95% CI, 0.77%–0.95%). On univariate analysis, no single vascular risk factor was associated with the presence of poor rLMCs (P ≥ .20 for all comparisons). In multivariable analysis, the rLMC score (good versus poor: OR, 16.7; 95% CI, 2.9%–97.4%; medium versus poor: OR, 9.2, 95% CI, 1.7%–50.6%), age (<80 years), baseline ASPECTS (≥8), and clot burden score (≥8) were independent predictors of good clinical outcome.
CONCLUSIONS: The rLMC score is a strong imaging parameter on CT angiography for predicting clinical outcomes in patients with acute ischemic strokes.
Abbreviations
- ACA
- anterior cerebral artery
- ASPECTS
- Alberta Stroke Program Early CT Score
- CI
- confidence interval
- CL
- confidence limit
- CTA
- CT angiography
- CTAsi
- CT angiography source image(s)
- DSA
- digital subtraction angiography
- IA
- intra-arterial
- ICA
- internal carotid artery
- IQR
- interquartile range
- IV
- intravenous
- MCA
- middle cerebral artery
- mRS
- modified Rankin Scale
- NCCT
- noncontrast CT
- NIHSS
- National Institutes of Health Stroke Scale score
- OR
- odds ratio
- PCA
- posterior cerebral artery
- rLMC
- regional leptomeningeal collateral
- TIA
- transient ischemic attack
- TOAST
- Trial of Org 10172 in Acute Stroke Treatment
- tPA
- tissue plasminogen activator
In the setting of acute ischemic stroke, therapeutic decision making is greatly influenced by determination of prognosis for a potentially disabling neurologic deficit, how much brain is at risk, and how much is salvageable. This is an imprecise and challenging process based on information extracted from clinical and radiologic assessments. There is a continued search to find better radiologic surrogates that enhance the accuracy of this determination.
Survival of brain tissue distal to an arterial occlusion is dependent on the status of collateral pathways, and for any occlusion distal to the circle of Willis, tissue viability is dependent on blood flow via leptomeningeal collateral pathways.1–3 Leptomeningeal collateral pathways consist of direct arteriolar anastomoses between pial branches of major cerebral arteries.4,5 These arteriolar connections contribute to retrograde filling of pial arteries distal to an occlusion. In MCA occlusions, leptomeningeal collateral scores by using DSA6,7 and CTA8–10 emphasize the anatomic extent of opacification of MCA branches, with excellent scores showing flow of contrast right to the distal end of the occluding thrombus.
Leptomeningeal collateral scoring systems based on CTA have been shown to correlate with and predict clinical outcome.7–10 These scores however either compare prominence of Sylvian sulcus arteries to the contralateral side or assess retrograde filling arteries in a “gestaltian” manner, without taking into account considerable regional variability in the presence of these arteries. Assessment of these backfilling arteries is also a temporal phenomenon as is image acquisition with CTA. Defining an optimal scan is therefore essential if scoring of collaterals is to be standardized. We have developed an rLMC score by using multiplanar reformatted CTA based on the major anatomic regions of the anterior circulation that is comparable to the ASPECTS method of scoring head CT11 in an attempt to improve on these deficiencies and to better predict tissue fate in the setting of acute ischemic stroke with an M1 MCA+/− intracranial ICA occlusion. We tested this score for interrater reliability and ability to predict clinical and imaging outcomes.
Materials and Methods
From an institutional review board–approved retrospective study of 1240 subjects presenting with acute ischemic stroke/TIA at our center from 2003 to 2009 and undergoing CTA, 159 patients with M1 MCA+/− intracranial ICA occlusion were included in the present study. Patients with incomplete M1 MCA occlusions were excluded because the degree of backfilling from collateral flow cannot be distinguished from forward flow in such cases. Patients with cervical ICA stenosis or occlusion but without an intracranial occlusion were also excluded.
Two authors (M.G. and B.K.M.) reviewed and scored by consensus baseline CT scans and CTA, blinded to clinical information and follow-up scans. ASPECTS is a 10-point scale to score early ischemic changes in MCA strokes. Baseline NCCT scan and CTAsi were scored for early ischemic changes and reduced contrast opacification, respectively, by using the ASPECTS system.11 Clot burden score was calculated on the CTA. This is a 10-point score assessing extent of clot in the proximal arteries of the anterior circulation.12 A higher score indicates lesser clot burden. Demographic data, vascular risk factors, stroke symptom onset time (last seen normal time in patients without witnessed stroke), CT scan time, and a 3-month mRS were collected from review of charts.
Imaging
During the study period (2003–2009), different multisection scanners (with detector numbers ranging from 4 initially to 64 in the latest scanners) were used. Standard nonhelical NCCT was performed on a multisection scanner (GE Healthcare, Milwaukee, Wisconsin or Siemens, Erlangen, Germany) using 120 kV, 170 mAs with 5-mm section thickness. NCCT was followed by CTA with a helical scan technique. Coverage was from arch to vertex with continuous axial sections parallel to the orbitomeatal line with 0.6–1.25-mm section thickness. Acquisitions were obtained after a single bolus IV contrast injection of 90–120 mL nonionic contrast media into an antecubital vein at 3–5 mL/s, autotriggered by appearance of contrast in a region of interest manually placed in the ascending aorta. In head only studies, minimum coverage was from foramen magnum to centrum semiovale. OsiriX version 3.5 (http://www.osirix-viewer.com), an image processing software designed for multiplanar reconstruction and volume rendering, was used to reconstruct 2D multiplanar reconstruction images in axial, coronal, and sagittal planes by using 40-mm-thick slabs. rLMC scores were obtained on these images (Fig 1A–C).
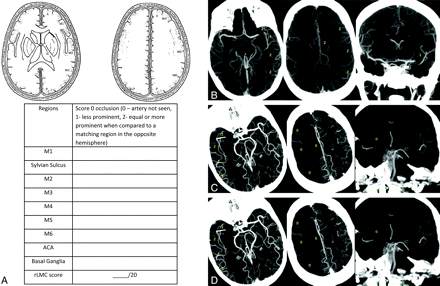
A, rLMC score is based on scoring pial and lenticulostriate arteries (0, no; 1, less; 2, equal or more prominent compared with matching region in opposite hemisphere) in 6 ASPECTS regions (M1–6) plus anterior cerebral artery region and basal ganglia. Pial arteries in the Sylvian sulcus are scored 0, 2, or 4. B, Left M1 MCA occlusion with prominent retrograde opacification of the pial arteries to the distal end of thrombus. rLMC score is 19. C, Right carotid “T occlusion” with patent ipsilateral A2 ACA segment and poor visualization of pial arteries in the right frontal and parietal regions. Note backfilling of pial arteries in the Sylvian sulcus with prominent well-visualized arteries in the temporal regions. Assessment of collateral status based on comparison of arteries in Sylvian sulcus alone suggests good PCA to MCA collaterals in the temporal regions and does not account for the poor PCA to ACA and ACA to MCA collaterals in the frontoparietal regions. rLMC score is 8. D, Left M1 MCA occlusion with poor leptomeningeal collateral status. All regions have less prominent or absent arteries. rLMC score is 7.
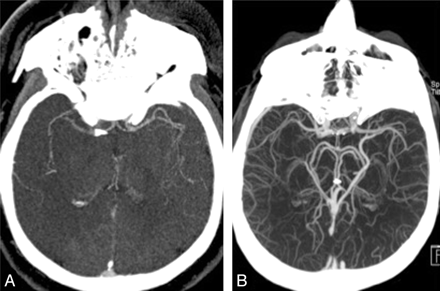
Scans were considered optimal for scoring collaterals if they included complete coverage from base of skull to vertex, clear visualization of arterial phase, and internal cerebral veins and dural sinuses in the normal hemisphere. In our experience, this ensures adequate time for retrograde opacification of the leptomeningeal collateral–dependent slower filling MCA branches distal to the M1 occlusion. We excluded 12 patients with scans showing inadequate contrast opacification of pial arteries on the normal side (Fig 2A). This could be either due to early triggering of the scan due to wrong placement of the region of interest in the pulmonary artery, contrast extravasation from the intravenous access site, or patient factors such as poor cardiac output. These factors result in insufficient circulation time for opacification of MCA vessels on the involved side and can underestimate collateral status. We also excluded 5 patients with scans where contrast opacification in the major cerebral veins (internal cerebral vein, basal vein of Rosenthal, or middle cerebral vein) on the side with arterial occlusion exceeded arterial opacification, ie, scans acquired primarily during the venous phase (Fig 2B). This could result in overestimation of collateral status compared with a scan acquired in the capillary or early venous phase. In addition, there could be difficulties in differentiating pial arteries from veins. In our experience, this type of scan is a result of delayed triggering or slow scanner (4- or 16-section versus 40- or 64-section scanner). All patients included in this study had complete M1 occlusions.
A, CTA showing occlusion of distal right M1 MCA. Poor contrast opacification of pial arteries even on the normal side (left) makes estimation of leptomeningeal collateral status difficult. B, Greater contrast opacification of the ipsilateral basal vein of Rosenthal than the MCA in a patient with occlusion of the distal left M1 MCA. Delayed triggering as evidenced by excessive venous contamination can result in overestimating collateral status.
rLMC Score
rLMC score (20 points) is based on scoring extent of contrast opacification in arteries distal to an M1 MCA+/− ICA occlusion (0, artery not seen; 1, less prominent; 2, equal or more prominent compared with a matching region in the opposite hemisphere) in the 6 ASPECTS cortical regions (M1–6), parasagittal ACA territory, and the basal ganglia (Fig 1A). Lenticulostriate arteries in the basal ganglia arising from retrograde filling MCAs distal to an occlusion are included in the scoring. Arteries in the Sylvian sulcus are given a higher score, ie, 0, 2, or 4 (0, not seen; 2; less; 4, same or prominent compared with the opposite Sylvian sulcus) because opacification of these vessels most distant from leptomeningeal ACA to MCA and PCA to MCA anastomoses is a strong indicator of good retrograde flow via these collateral networks. Higher total scores indicate better collateral status (Fig 1B–D). Interrater reliability was determined on 15 scans included in the study by 2 independent raters (B.K.M. and J.M.) and was excellent (intraclass correlation coefficient, 0.87; 95% CI, 0.77%–0.95%).
Statistics
Primary outcome was mRS ≤2 at 3 months. rLMC score was analyzed as a continuous variable or trichotomized into 0–10, 11–16, and 17–20 for analysis. Univariate comparisons were by Fisher exact test, Wilcoxon rank sum test, or Kruskal-Wallis test, as appropriate. Univariate correlations between the rLMC score and baseline NIHSS, NCCT ASPECTS, CTAsi ASPECTS, follow-up CT ASPECTS, clot burden score, and mRS at 3 months were determined by Spearman correlation coefficients. Logistic regression models with backward elimination of nonsignificant variables (P > .05) were used to identify variables independently associated with poor collateral score (0–10) and primary clinical outcome. Only main effects were considered. ASPECTS was dichotomized into >7 versus ≤7 and clot burden score into 0–5 versus 6–10 for these analyses, as in previous studies.12,13 Variables were chosen for the model based on clinical knowledge or an association with the outcome in univariate analysis (P ≤ .15). Van Elteren test was used to test the relationship between collateral category and time from symptom onset to CTA, stratified by median NIHSS. Cochran-Mantel-Haenszel test with Breslow-Day test for homogeneity of ORs were used to test prespecified hypotheses that relationships between intra-arterial therapy and good clinical outcome, and baseline ASPECTS score and good clinical outcome are modified by collateral score category. Statistical analyses were conducted by using SAS version 9.2 (SAS Institute, Cary, North Carolina). All tests were 2-tailed, and conventional levels of statistical significance were used (α = 0.05).
Results
Excluding patients without optimal imaging (n = 17) and missing clinical data (n = 4), 138 patients (64 males; median baseline NIHSS, 16) were included in the analysis. Overall, 37.6% had good (17–20), 40.5% had medium (11–16), and 21.7% had poor (0–10) rLMC scores. Median stroke onset to CTA time was 164 minutes; most patients (75%) were scanned at 361 or fewer minutes after stroke onset. Other patient characteristics, grouped according to the presence or absence of good clinical outcome, are shown in On-line Table 1.
Correlation between rLMC Score and Baseline and Follow-Up Imaging Parameters
Better collateral status as assessed by a higher rLMC score showed weak correlation with higher baseline NCCT ASPECTS (Spearman r = 0.25; 95% CI, 0.09%–0.40%; P = .003) but demonstrated strong correlation with higher follow-up CT ASPECTS (Spearman r = 0.58; 95% CI, 0.46%–0.68%; P < .001). A moderate correlation was found with higher baseline CTAsi ASPECTS (Spearman r = 0.50; 95% CI, 0.36%–0.62%; P < .001). A higher rLMC score also correlated with a higher clot burden score (Spearman r = 0.26; 95% CI, 0.09%–0.40%; P = .003).
Variables Associated with Collateral Flow as Assessed by a Trichotomized rLMC Score (17–20, 11–16, and 0–10)
On univariate analysis, no single vascular risk factor (including age, sex, smoking status, diabetes mellitus, hypertension, coronary artery disease, and previous stroke or TIA) was associated with the presence of poor rLMCs (P ≥ .20 for all comparisons; data not shown). Baseline NIHSS (P = .01), NCCT ASPECTS (P < .001), CTAsi ASPECTS (P < .001), and clot burden score (P < .001) were associated with the rLMC score category. Median time from stroke onset to CTA was 124 minutes in patients with poor collaterals (IQR, 81–201), 176 minutes in patients with medium collaterals (IQR, 97–444), and 173 minutes in patients with good collaterals (IQR, 93–405) (P = .25 by Kruskal-Wallis test). Because higher stroke severity is associated with earlier time of presentation and worse collaterals, we also tested the relationship between collateral category and time from symptom onset to scan, stratified by median NIHSS, and we found no relationship (P = .45 by van Elteren test). In multivariable analysis, only baseline NIHSS (OR, 1.1 per 1-point increase in NIHSS; 95% CI, 1.0%–1.2%; P = .04) and baseline CTAsi ASPECTS (OR, 0.08 when CTAsi >7; 95% CI, 0.02%–0.3%; P = .001) were associated with poor collateral score.
Variables Associated with Good Clinical Outcome mRS ≤2
Of 138 patients included in the study, 5 with baseline mRS >2 were excluded from outcome analysis. Higher rLMC score was significantly correlated with lower baseline NIHSS (Spearman r = −0.36; 95% CI, −0.50% to −0.21%; P < .001) and with lower mRS at 3 months (Spearman r = −0.47; 95% CI, −0.59% to −0.33%; P < .001). Good clinical outcome was seen in 52% of patients with good rLMC score (17–20), 34% with medium rLMC score (11–16), and 7% with poor rLMC score (0–10) (P < .001). In univariate analysis, higher rLMC score, lower age, lower baseline NIHSS, higher baseline ASPECTS and CTAsi ASPECTS, higher clot burden score, and any endovascular therapy (IA alone or IV + IA tPA or mechanical) were associated with good clinical outcome (On-line Table 1). In multivariable analysis, rLMC score (good versus poor: OR, 16.7; 95% CI, 2.9%–97.4%; medium versus poor: OR, 9.2; 95% CI, 1.7%–50.6%), age (<80 years), baseline ASPECTS (≥8), and clot burden score (≥8) were independent predictors of good clinical outcome (On-line Table 2). When the presence or absence of proximal ICA stenosis (>70%)/occlusion was added to the model (data available in 130/133 subjects), the relationship between rLMC score and outcome remained highly significant (data not shown).
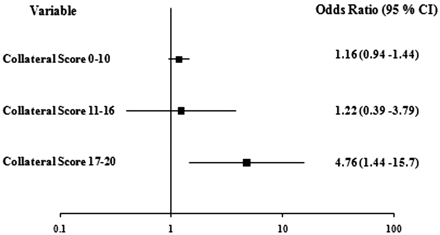
We hypothesized that patients with better collateral score are more likely to have a good clinical outcome when baseline ASPECTS was high (>7) versus low (0–7) and when endovascular therapy was performed. We failed to find evidence of a different relationship between baseline ASPECTS and good clinical outcome in the presence or absence of better collaterals (P = .52). In the group of patients with good collateral scores, endovascular therapy was associated with good clinical outcome (OR, 4.76; 95% CI, 1.44%–15.7%), though a test failed to show significant heterogeneity of the ORs in the different collateral score categories (P = .16).
Discussion
Leptomeningeal collaterals are direct arteriolo-arteriolar connections between major cerebral arteries that provide a route for retrograde filling of pial arteries distal to an occluded artery. They provide a vascular network with the potential to maintain cerebral blood flow at levels that prolong or indefinitely sustain brain tissue viability beyond an occlusion.14 Good flow through collateral pathways is associated with a larger penumbra and smaller infarct core at baseline15,16 and by extending the survival time of penumbra, can extend the time window for viable reperfusion. Good collaterals therefore limit infarct core expansion and determine final infarct volumes.
We describe a novel method of scoring leptomeningeal collaterals based on the extent of opacification of pial and lenticulostriate arteries distal to an M1 MCA+/− intracranial ICA occlusion on CTA. The rLMC score is a semiquantitative system of scoring based on the major anatomic regions of the anterior circulation and is comparable to the ASPECTS method of scoring head CTs.11 We have demonstrated a strong correlation between the rLMC score at baseline and both radiologic and clinical outcomes. The rLMC score has high interrater reliability (interclass correlation coefficient, 0.87; 95% CI, 0.77%–0.95%) and is strongly correlated with follow-up NCCT ASPECTS in our study. In addition, we are able to show in a multivariable analysis that CTAsi does not carry any independent information about clinical outcome when information about baseline NCCT and collateral status are available. Moderate correlation (Spearman r = 0.50; 95% CI, 0.36%–0.62%; P < .001) is a possible explanation. A biologic explanation could be that reduced contrast opacification seen on CTAsi that is not already seen on NCCT is probably tissue-dependent on collateral flow; therefore, when information on NCCT ASPECTS and collateral scores is available, CTAsi does not have any additional information. Our results are in agreement with previous studies that show collateral status to be an important determinant of final infarct core.7,17
Our study shows that collateral status is a strong independent predictor of clinical outcome. Kim et al17 have shown previously by using DSA that a regional angiographic score correlates significantly with final infarct and has higher interobserver reliability. Although other CTA-based studies have shown that collateral status predicts clinical outcomes,8,10 the rLMC score, being a less subjective ordinal scale and focusing on all areas of anterior circulation, is able to show a stronger correlation. Infarct core or penumbra mismatch should intuitively correlate with collateral status. Miteff et al16 showed that 65.4% of patients with a mismatch ratio ≥3 have good collateral status. Along with Bang et al,15 they suggest that collateral status may better define degree of hypoperfusion in areas within prespecified penumbral thresholds. We have been able to show that the rLMC score correlates strongly with size of infarct core at baseline and is a strong independent predictor of final infarct and clinical outcome. Multivariable analyses of our data show that collateral status, size of infarct core, and clot burden are the 3 most important imaging variables predicting final infarct and clinical outcome along with age (On-line Table 2). These variables give an estimate of irreversibly injured and salvageable brain tissue along with thrombus load in the arterial tree and thus the probability of good clinical outcome. It is therefore possible that the infarct core (as measured by the ASPECTS system), along with a trichotomized rLMC score, and the clot burden score may provide a simple alternative to “core and penumbral mismatch” and allow easier decision making in the management of patients with acute ischemic strokes and proximal vessel occlusions. We do however recognize that the retrospective nature of our study is a limitation and believe that a prospectively designed study will be able to address this issue better. We do not find that baseline NIHSS predicts clinical outcome. We therefore confirm similar findings reported by Maas et al.10 More than 75% of our patients have a baseline NIHSS >10, ie, have moderate-to-severe strokes, and all have M1+/− intracranial ICA occlusions. In such a patient cohort in which the natural history of the disease would lead to poor outcomes without treatment, baseline NIHSS may not correlate with clinical outcomes when adjusting for therapy. As Maas et al10 suggest, that clinical worsening is significantly more common in patients with poor collateral status also could be a reason why NIHSS at baseline does not predict clinical outcome.
We did not find a relationship between rLMC and onset to CTA time. A modest trend toward shorter onset to CTA times in patients with poor collaterals was less apparent when controlling for higher stroke severity, which was associated with both shorter time to scan and worse collateral scores. Our findings are consistent with other studies showing that time to imaging correlates poorly with collateral status.7,16 As in other studies, we substituted the time the patient was last seen normal when the exact onset time was unknown. This is a potential limitation of all analyses in acute stroke. Most patients in our study, however, were scanned within 6 hours of stroke onset. We also had little power to detect a relationship between collateral failure and time, if collaterals tend to fail only after ≥6 hours. We acknowledge that collaterals may “fail” in tissue regions that subsequently progress to infarction. Collateral status however need not worsen over time for ischemia to progress to infarction. An improved understanding of the temporal relationships between collateral status and tissue fate will require serial imaging over time, including at time points later than 6 hours.
Factors determining collateral status are mostly unknown. Genetic variability has been shown to be a major determinant of collateral status in mice.18 Other studies show the effect of cytokines such as vascular endothelial growth factor,19 granulocyte macrophage–colony-stimulating factor 20; angiotensin (AT1) receptor blockade,21 catecholamines,22 and pCO2 and blood pressure23 in determining collateral status. The dilatory capacity of small interarteriolar connections in other vascular beds is impaired by raised blood glucose, diabetic status, hypertension, and smoking, factors that affect endothelial function.20,24 Our study shows that no single vascular risk factor, including smoking, diabetes, hypertension, coronary arterial disease, or previous stroke or TIA, predicts collateral status in acute ischemic strokes. Old age correlates with decreased branching attenuation in the microcirculation.25 We have, however, not been able to show a correlation between age and collateral status.
Our study suggests that there may be an association between endovascular therapy and good outcome in patients with excellent collateral scores (Fig 3). The difference in the strength of the association across collateral score categories, however, showed only a trend toward significance; therefore, these findings warrant confirmation in other studies. Nonetheless, the association is biologically plausible.
Relationship between IA therapy and good outcome according to collateral score category (n = 133; 5 were excluded for baseline mRS >2). Cochran-Mantel-Haenszel test for homogeneity of ORs, P = .16.
A limitation of scoring collaterals by using CTA is significant proximal stenosis or occlusion or a scan acquisition triggered early. This may lead to nonvisualization of leptomeningeal arteries due to longer transit time for blood flow across the stenosis or occlusion in the former case or insufficient time for contrast to occur in the pial arteries in the latter case. Unlike CT perfusion studies in which delay correction is used,26 CTA does not permit similar use. Despite use of an autotriggering protocol, we identified several patients with possible early or delayed triggering, probably related to technical errors or intrinsic patient factors. To date few attempts has been made to calculate rate of collateral filling with either DSA or CTA and previous studies on leptomeningeal collaterals suffer from these limitations.8–10 By applying a standard protocol based on inspection of the degree of opacification of the arterial and venous structures, we have tried to reduce some of these potential errors. The regional nature of our scoring system and application of strict imaging-based exclusion criteria to account for contrast bolus timing–related issues strengthens our study in comparison with previous studies. The retrospective nature of data collection, some patients being excluded due to not getting CTA, and the lack of reperfusion data in many of our patients are potential drawbacks. Also, we note that patients with incomplete occlusion were by design not included in our study, because the degree of backfilling from collateral flow cannot be distinguished from forward flow in such cases; therefore, our score may not be applicable in such patients. Newer dynamic and time-resolved CTA techniques could address some of these limitations in future.
Conclusions
Collateral status is an important biologic determinant of clinical and imaging outcomes in acute ischemic stroke. Factors responsible for variability in collateral status are still unknown. A reliable and easy-to-use CTA-based rLMC score could be useful in predicting outcomes and making therapeutic decisions in acute ischemic stroke. It also could be an imaging (biologic) outcome in future trials aimed at enhancing collaterals by various interventions.
Footnotes
-
Disclosures: Eric Smith, Consultant: Genentech. Details: Consultant 2010–2011; <$2,000, Michael Hill, Research Support (including provision of equipment or materials): Hoffmann-LaRoche Canada, Ltd. Details: Drug-in-kind support for research study. Consultant: Calgary Scientific Inc. Details: No financial remuneration. Testing of technology. Ownership Interest: Calgary Scientific Inc. Details: Common stock. Private investment. Amount: significant. Mayank Goyal, Consultant: Calgary Scientific, Inc. Andrew M. Demchuk, Consultant: Calgary Scientific, Inc.
References
- Received October 9, 2010.
- Accepted after revision January 18, 2011.
- © 2011 by American Journal of Neuroradiology