Abstract
SUMMARY: 1H-MR spectroscopy is an established noninvasive MR imaging technique that can be helpful in the diagnosis of brain lesions and in treatment planning. Claustrophobia and body habitus preclude some patients from routine MR imaging in a closed-bore system. The development of 1H-MR spectroscopy for use in an open MR imaging system would enable a more complete characterization of brain lesions in these patients.
Abbreviations
- CH2
- methyl
- Cho
- choline
- Cr
- creatine
- FLAIR
- fluid-attenuated inversion recovery
- 1H-MR spectroscopy
- proton MR spectroscopy
- NAA
- N-acetylaspartate
- PML
- progressive multifocal leukoencephalopathy
- PRESS
- point-resolved spectroscopic sequence
- SNR
- signal-to-noise ratio
- SVS
- single-voxel spectroscopy
1H-MR spectroscopy is a useful noninvasive MR imaging technique that aids in the diagnosis of brain lesions before biopsy, and more recently has been used for biopsy targeting in heterogeneous tumors, evaluation of change of tumors with time, and treatment planning.1–3 Biopsy can sometimes be avoided altogether if imaging and 1H-MR spectroscopy can rule out certain diagnoses, and this change is significant with reported morbidity of biopsies being 3%–4%.4 Moller-Hartmann et al5 showed that the addition of 1H-MR spectroscopy compared with MR imaging alone provides a 15.4% increase in correctly diagnosing a lesion and a 6.2% reduction in misdiagnosing a lesion. Patient body habitus and claustrophobia may limit some patients from the possible benefits of metabolic information gleaned from 1H-MR spectroscopy imaging in a closed-bore magnet at 1.5T or 3T. Recent feasibility studies have shown that reliable spectra can be obtained on a commercially available 1T open MR imaging system.6,7 In this technical report, we present preliminary data on the clinical use of 1H-MR spectroscopy for use in open MR imaging at 1T.
Technique
Approval was obtained for this study from the institutional review board of the University of Vermont. Water-suppressed SVS of the brain was performed on a high-field open Panorama 1T MR imaging scanner (Philips Healthcare, Best, the Netherlands) by using PRESS localization with a 4-channel solenoid technology SENSE coil. PRESS SVS was added to routine clinical MR imaging examinations either with contrast injection (3-plane T1-weighted turbo spin-echo pre- and postcontrast; axial T2-weighted turbo spin-echo; T2-weighted FLAIR; T2-weighted gradient-echo; and diffusion-weighted imaging, total scan-session duration = 31 minutes) or without contrast injection (sagittal T1-weighted FLAIR instead of 3-plane pre- and postcontrast, total scan-session duration = 20 minutes). Spectra were acquired on 9 patients (5 women, 4 men; 28–78 years of age; mean age, 42 years). Voxels were placed in the centrum semiovale bilaterally in patients with normal-appearing brain on MR imaging. All 9 of these patients had a clinical indication of headache, and the results of the brain MR imaging examinations were interpreted as normal for patient age by a board-certified neuroradiologist with certificate of added qualification. The centrum semiovale was chosen given institutional experience with acquiring normative data on 1H-MR spectroscopy at 1.5T and 3T within the centrum semiovale.
Two patients who failed imaging at 3T due to claustrophobia and who had intracranial mass lesions were studied in the 1T open MR imaging scanner with PRESS SVS. One was a 38-year-old man with a cystic enhancing lesion in the right frontal lobe with focal mass effect and slight shift from right to left. He had presented with headache to his primary care physician who ordered the MR imaging examination. A voxel was placed over the intracranial mass lesion, and he became claustrophobic and could not tolerate a voxel placed on the contralateral side. The second patient was an immunocompromised 51-year-old man with a CD4 count of 14, who presented with decreased cognition to his primary care physician, who ordered MR imaging. Brain MR imaging showed a lesion in the right temporal subcortical white matter without focal mass effect or enhancement. Voxels were placed over the lesion and over the contralateral normal-appearing left temporal subcortical white matter.
Data were acquired with TR/TE of 1500/144 ms with SVS voxel sizes of 2 cm3 with 192 signal-intensity averages with 1-kHz sampling bandwidth and an acquisition time of 4 minutes, 48 seconds. Taking advantage of open magnet configuration, we performed automatic left-to-right and superior-to-inferior table movements, so that voxels were acquired at the magnet isocenter. Spectroscopic features available to the technologist for this system included all those available for a 1.5T MR imaging scanner (eg, outer volume suppression, spectroscopic imaging). While the manufacturer-supplied protocol has a voxel size of 15 × 15 × 15 mm, a voxel as small as 10 × 10 × 10 mm is possible with the available gradients; for this study however, the manufacturer-recommended voxel size was used on the basis of initial trials to acquire adequate spectral SNR within 5 minutes. Automatic shimming was performed in all cases, and the mean of the full width at half maximum for the water peak averaged over all subjects was 0.10 ppm (4 Hz). For water suppression, a train of four 50-ms narrowband pulses was applied before the excitation pulse in a manner similar to the water suppression through enhanced T1 effects (WET) technique.8 The pulse shapes and flip angles were optimized to minimize sensitivity to the T1 of water and to the overall radio-frequency field strength.9 To avoid inadvertent saturation of metabolites close to water, we shifted the center of the water suppression notch 1 ppm downfield, away from the peaks of interest. As a result of this frequency-shift strategy, the CH2 peak of creatine at 3.92 ppm was clearly present in all spectra. All spectra were processed with SpectroView (Philips Healthcare): eddy current correction by using an unsuppressed water reference signal-intensity time-domain filtering, fast Fourier transform, phase correction, Marquardt-Levenberg nonlinear least squares fitting of peaks and baseline by using prior knowledge of expected metabolites, and subtraction of the fitted baseline. NAA/Cr and Cho/Cr ratios were recorded along with presence or absence of lactate peaks. Absolute metabolite concentrations were not determined.
Results
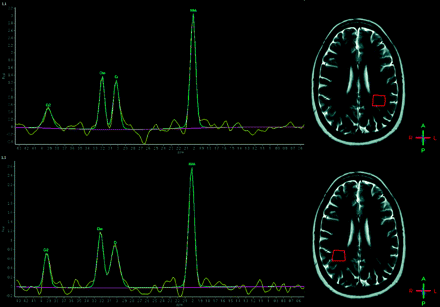
Nine patients who had brain MR imaging examinations interpreted as normal for patient age had the following 1H-MR spectroscopy values within the right centrum semiovale: NAA/Cr = 2.38 ± 0.31 and Cho/Cr = 1.18 ± 0.11. Average 1H-MR spectroscopy measurements in the left centrum semiovale were the following: NAA/Cr = 2.42 ± 0.23 and Cho/Cr = 1.13 ± 0.09. Bilaterally no lactate or lipids were detected (Fig 1).
A healthy 28-year-old woman, free of pathology. Top: NAA/Cr = 2.34, Cho/Cr = 1.10, NAA/Cho = 2.13, Cho/NAA = 0.47. Bottom: NAA/Cr = 2.88, Cho/Cr = 1.32, NAA/Cho = 2.18, Cho/NAA = 0.46.
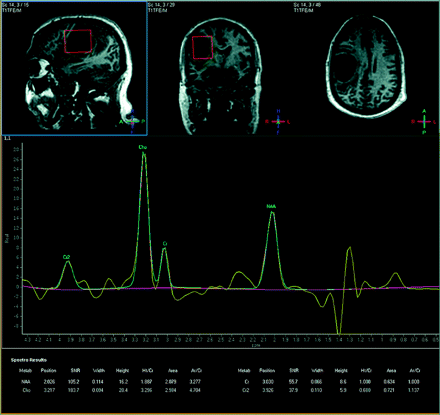
For the 38-year-old man with a cystic enhancing lesion in the right frontal lobe with focal mass effect and slight shift from right to left, 1H-MR spectroscopy revealed an NAA/Cr ratio of 1.89 and a Cho/Cr ratio of 3.30, with both lactate and lipid peaks present (Fig 2). Findings were suggestive of a high-grade glial neoplasm, especially given the elevated Cho/Cr, and a diagnosis of a glioblastoma multiforme was confirmed postimaging by biopsy.
A 38-year-old man with glioblastoma multiforme as confirmed by biopsy. NAA/Cr = 1.89, Cho/Cr = 3.30, NAA/Cho = 0.57, Cho/NAA = 1.75.
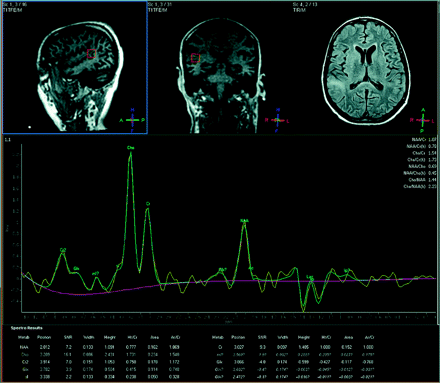
The second patient underwent 1H-MR spectroscopy, which revealed an NAA/Cr ratio of 0.77 and a Cho/Cr ratio of 1.73, with a lactate peak (Fig 3). 1H-MR spectroscopy and imaging findings suggested PML, which was confirmed with JC virus titers in the CSF.
Patient with PML (voxel = 15 × 15 × 15 mm). NAA/Cr = 0.78, Cho/Cr = 1.73, NAA/Cho = 0.45, Cho/NAA = 2.23.
The SNR of the water peak varied with partial volume pathology, but the mean SNR was 3522 for a 15 × 15 × 15 mm voxel (calculated by SpectroView in the frequency-domain as [peak height] / [2 × σ] after filtering).
Discussion
The preliminary results on the 9 patients with normal-appearing brain MR imaging examinations and the 2 patients with intracranial mass lesions showed that reliable diagnostic spectra can be obtained on an open MR imaging system at 1T. Although there continues to be a trend toward the use of higher field strength magnet systems at 3T in diagnostic neuroradiology, there is a parallel trend in a growing number of habitus-limited MR imaging studies due to increasing rates of obesity in adults and children as well as studies terminated due to patient claustrophobia.10 Given the utility of 1H-MR spectroscopy in the diagnosis of brain lesions and as a potential tool for biopsy targeting, monitoring tumor changes with time, and treatment planning,1–3 there is a real clinical value in the development of a reliable 1H-MR spectroscopy sequence for use on an open-magnet system at 1T, so that patients with claustrophobia or obesity will not have impaired access to imaging. In both of the clinical cases showing intracranial mass lesions, the 1H-MR spectroscopy sequence was diagnostic in quality and helped with differential diagnosis. In the large cystic mass in the right frontal lobe, the Cho/Cr ratio and lactate peak suggested a high-grade neoplasm like glioblastoma multiforme.11,12 In the second case, the decrease in NAA/Cr with the corresponding increase in Cho/Cr ratio and the presence of a lactate peak in the setting of a subcortical white matter lesion without enhancement or mass effect strongly suggested PML in this patient with end-stage acquired immunodeficiency syndrome.13,14
There are both technical disadvantages and advantages provided by the system used in this study. SNR is generally lower for lower magnetic field strengths (B0). Due to the vertical orientation of the field and the open-magnet shape however, SNR can be significantly improved by the use of a solenoid receive coil. Another obstacle is the fact that peak separation is reduced at a lower B0, thus potentially making spectral peak overlap and water suppression more of a challenge. On the basis of the measured peak widths and the flat baseline, this obstacle was less of a problem than initially suspected, indicating acceptable performance of the shim calibration. While the system lacks high-order shims because of the lateral table motion possible in an open magnet system, the voxel can be moved laterally to the most homogeneous place in the magnetic field. A clear advantage seen at a lower B0 is the reduction of both chemical shift misregistration and the effects of susceptibility differences. Consequently, one can more easily place a spectroscopic voxel closer to the scalp or skull with less line-shape distortion and less unwanted lipid signal intensity from subcutaneous fat. Another advantage, potentially, is the fact that line shapes for strongly J-coupled metabolites such as glutamate and myo-inositol are more coalesced at lower field strengths and are thus more easily detected.
Even though this is only a small sample size, 1T open 1H-MR spectroscopy does show promise. Some patients cannot undergo a closed-bore MR imaging examination, due to claustrophobia or body habitus, which is an increasing concern given the increased rates of obesity in pediatric and adult patients. Therefore, there is a real clinical value in the development of a reliable 1H-MR spectroscopy sequence for use in open MR imaging, so that these patients have access to the potential benefits of metabolic imaging with 1H-MR spectroscopy, which is a useful adjunct to routine brain MR imaging and allows a more accurate diagnosis of brain lesions and potential use in biopsy targeting and treatment planning. Future research directions involving 1T open MR spectroscopy may include a larger patient series or direct comparisons of 1H-MR spectroscopy data from 1 patient population by using both 1T open 1H-MR spectroscopy and closed-bore high-field (1.5T or 3T) 1H-MR spectroscopy. Despite the greater difficulty in shimming over the multivoxel PRESS box, spectroscopic imaging has been successfully performed on the 1T open MR imaging system,8 and a more complete presentation of this work will be forthcoming.
References
- Received February 1, 2010.
- Accepted after revision July 31, 2010.
- © 2011 by American Journal of Neuroradiology