Abstract
BACKGROUND AND PURPOSE: Previous studies evaluating vertebral augmentation procedure costs have not made detailed comparisons between vertebroplasty and kyphoplasty. Our study contrasts hospital costs for vertebroplasty versus kyphoplasty for the treatment of vertebral compression fractures in routine clinical practice in the United States.
MATERIALS AND METHODS: This retrospective cohort study analyzed 2007–2008 hospital discharge and billing records from the Premier Perspective data base. The primary outcome variable, differences in total hospital cost between vertebroplasty and kyphoplasty, was assessed by using analysis of covariance.
RESULTS: Three thousand six hundred seventeen patients received vertebroplasty (64% inpatient, 36% outpatient), and 8118 received kyphoplasty (54% inpatient, 46% outpatient). Approximately 75% were women, and most were white. Mean total unadjusted inpatient costs were $9837 for vertebroplasty versus $13 187 for kyphoplasty (P < .0001). Outpatient vertebroplasty costs were $3319 versus $8100 for kyphoplasty (P < .0001). Lower vertebroplasty costs were largely due to differences in hospital supply and OR. Mean vertebroplasty OR costs were $73.60 (anesthesia), $112.06 (recovery room), and $990.12 (surgery) versus $172.16 (anesthesia), $257.47 (recovery room), and $1,471.49 (surgery) with kyphoplasty. Adjustments for age, sex, admission status, and disease severity accentuated the differences. Mean adjusted inpatient costs were $11 386 for vertebroplasty versus $16 182 for kyphoplasty (P < .0001), and outpatient costs were $2997 for vertebroplasty versus $7010 for kyphoplasty (P < .0001). After adjustments for the same covariates, length-of-stay differences were no longer evident (P = .4945).
CONCLUSIONS: Performing vertebroplasty versus kyphoplasty reduces hospital costs by nearly $5000 for inpatient procedures and by more than $4000 for outpatient procedures.
Abbreviations
- APR
- all-patient refined
- CAP
- capitated
- CPT
- Current Procedural Terminology
- DRG
- diagnosis related group
- ICD-9-CM
- International Classification of Diseases, ninth revision, Clinical Modification
- NA
- not applicable
- OR
- operating room
- VAP
- vertebral augmentation procedure
- VCF
- vertebral compression fracture
The clinical burden associated with bone pain and spinal axial deformity in VCF has been well documented in the literature.1,2 Traditional conservative medical management for VCFs has included bed rest, pain medications, and bracing. Surgical treatment has been an option of last resort because of long recovery, poor outcomes, and complication risk among patients of advanced age who commonly present with multiple comorbidities. Patients with painful VCFs can benefit from VAPs, which provide immediate pain relief and allow return to function with minimal stress on health. In the mid 1980s, vertebroplasty was introduced as a minimally invasive VAP for treatment of VCFs. Kyphoplasty, a variation of vertebroplasty, was subsequently introduced in the late 1990s. Given the prevalence of VCFs as well as demographic trends, the economics of VAPs is important from a public policy perspective. The purpose of this study was to contrast hospital economics for the treatment of VCFs with either vertebroplasty or kyphoplasty.
Several landmark studies of VAPs have been published since a 2007 multisociety consensus statement concluded that vertebroplasty and kyphoplasty are safe and efficacious for the treatment of VCFs and noted no proved advantages of kyphoplasty over vertebroplasty with respect to pain relief, vertebral height restoration, or complication rates.3 In the most recent randomized controlled clinical trial, patients who received vertebroplasty for treatment of acute VCFs experienced superior pain relief that was sustained for 1 year compared with patients who received conservative medical management.4 At the 1-year follow-up interval in a similarly designed study, patients who received kyphoplasty experienced superior improvements in quality of life versus their conservatively managed counterparts.5 The results of these studies contrast with those of 2 recent randomized controlled trials, in which improvements in pain and pain-related disability among patients who received vertebroplasty were similar to those among patients who received a simulated procedure without cement.6,7 The debate following these studies underscores the challenges in conducting adequately powered clinical trials for VAPs, including patient enrollment and management of patient crossover.8,9
Previous studies evaluated the costs associated with VAPs, though few made detailed cost comparisons between vertebroplasty and kyphoplasty. Lad et al (2009)10 assessed national trends by using data from the 2004 Nationwide Inpatient Sample. In that year, approximately 23 000 VAPs were performed nationwide with 60% of patients being women between the ages of 65 and 84. Unadjusted mean hospital charges for both procedures were comparable, and the total “national bill” was estimated to be $672 million. Gray et al (2008)11 evaluated charges associated with thoracolumbar vertebroplasties performed from 2001 to 2005 by using Medicare Part B fee-for-service claims data. Although total nationwide inflation-adjusted charges from inpatient and outpatient VAPs increased from $76.0 million for 14 142 cases performed in 2001 to $152.3 million for 29 090 cases in 2005, per-procedure costs decreased from $5374 to $5235. Inpatient cases generated most of the charges, though vertebroplasty was predominantly performed in the outpatient setting.
The cost-effectiveness of percutaneous vertebroplasty relative to conservative medical management was evaluated through a retrospective chart review of 179 patients.12 Significant reduction of pain and improvement in function were observed in both groups through 12 months postsurgery. Vertebroplasty was more cost-effective than conservative medical management at 1 week and 3 months (P < .05), though differences were not evident at 12 months.
Our study sought to contrast hospital costs for vertebroplasty versus kyphoplasty for the treatment of VCFs in routine clinical practice in the United States. Both hospital inpatient and outpatient resource use and costs were assessed. Unlike prior studies that relied on hospital charge data to evaluate the economics of VAPs, this study examined cost data derived from hospital-specific accounting systems or cost-to-charge ratios (total and departmental). Important components of costs, such as supply, OR, room and board, and radiology, were included in the analysis. Comparative analyses of costs and hospital length of stay included statistical adjustments to account for between-group differences in baseline patient characteristics.
Materials and Methods
This retrospective cohort study used hospital discharge and billing records from the Premier Perspective data base (http://www.premierinc.com/quality-safety/tools-services/prs/data/perspective.jsp), a data base developed for measuring quality and use of health care, representing >600 hospitals. Participating hospitals represent all regions of the United States and are predominantly small-to-midsize nonteaching facilities that serve largely urban patient populations. Hospitals within the Premier Perspective data base are self-selected. They pay a fee and are on contract with Premier Perspective to receive access to informatics tools and services. The hospitals submit data voluntarily, and their primary hospital characteristics are representative of those within the annual survey of hospitals in the United States by the American Hospital Association. Unlike individual insurance data bases, data within the Premier Perspective data base are not limited by payer status. Available data include admission and discharge characteristics, hospital characteristics, billing information, patient demographics, physician information, and cost and charge data. (The Premier dataset contains cost variables that reflect billable amounts and hospital costs. The cost component includes all supplies, labor, depreciation of equipment, and so forth. It is the sum of the fixed [overhead] and variable [direct] costs contained in the dataset.)
Data from 2007 to 2008 were analyzed. Inpatient hospitalizations with a primary ICD-9-CM (http://icd9cm.chrisendres.com/) procedure code for vertebroplasty (81.65) or percutaneous vertebral augmentation (81.66) and outpatient services with a primary ICD-9-CM procedure code and at least 1 vertebroplasty or kyphoplasty CPT code (22520–5) were analyzed (Table 1). Patients were excluded from the analysis if 1 of the following ICD-9-CM diagnosis codes suggestive of VCF was not evident during the index procedure: pathologic fracture of vertebrae (733.13, pathologic fracture as defined by ICD-9-CM includes fracture due to primary osteoporosis); closed fracture of lumbar vertebra without spinal cord injury (805.4); or closed fracture of dorsal (thoracic) vertebra without spinal cord injury (805.2). Patients with a cancer diagnosis, known multiple hospitalizations, or outpatient visits (in the same hospital system) for vertebroplasty or kyphoplasty and those who received both a vertebroplasty and kyphoplasty procedure during the same hospitalization were also excluded.
ICD-9-CM procedure and CPT procedure codes used for identification of the study cohorta
For each patient, the type of fracture, type of procedure (vertebroplasty or kyphoplasty), setting of care (inpatient or outpatient), admission status (elective or emergency), and hospital characteristics were identified. Patient characteristics included demographics (age, sex, race or ethnic group, and marital status), comorbidities, and patient severity (Charlson Comorbidity Index and APR-DRG severity/mortality indices). Patient severity was described by using both the Charlson Comorbidity Index and APR-DRG severity (inpatient only) constructs to capture 1-year predictive mortality and clinical complexity, respectively. We also evaluated the type of hospital (rural/urban and teaching/nonteaching), geographic region, payer, and hospital bed size. Data on in-hospital length of stay and hospital costs were analyzed.
The primary outcome variable, differences in total hospital cost between vertebroplasty and kyphoplasty, was assessed by using analysis of covariance. Statistical distributions for cost variables were evaluated to determine if transformations were needed to achieve normality. Interactions between the procedure indicator and the covariates were tested, and stratified models were used if interactions were significant at P < .05.
Investigation of component (departmental) hospital costs was exploratory, and no adjustments were made for multiple tests with these variables. Independent-sample t tests were used to assess between-group differences in component-specific direct medical costs incurred during the index vertebroplasty or kyphoplasty procedure. Analyses were carried out by using SAS software (Version 9.2; SAS Institute, Cary, North Carolina).
Results
A total of 21,221 patients had at least 1 of the identified ICD-9-CM primary procedure or CPT procedure codes in 2007 or 2008. The attrition of the sample is shown in Fig 1.
Sample attrition: number of patients meeting study criteria.
A total of 3617 patients received vertebroplasty (64% inpatient and 36% outpatient) and 8118 patients received kyphoplasty (54% inpatient and 46% outpatient) for treatment of VCF. Patients had mean age of 78 (vertebroplasty) and 76 (kyphoplasty) years. Approximately 75% of vertebroplasties and kyphoplasties was performed in women, and most patients were white. Charlson Comorbidity Index scores were higher in inpatients than in outpatients. More patients in the vertebroplasty group (9.5%) had an APR-DRG mortality rating of “major” or “extreme” than patients in the kyphoplasty group (5.3%). Among all patients, comorbidities considered to be predictive of mortality included atrial fibrillation, coronary arthrosclerosis, congestive heart failure, long-term anticoagulant use, and persistent mental disorders.
Pathologic fracture of vertebra (ICD-9-CM 733.13) was the primary diagnosis for approximately 50% of patients. Between 20% and 30% of patients had a primary diagnosis of closed fracture of lumbar vertebra without mention of spinal cord injury and a similar percentage of patients had a primary diagnosis of closed fracture of dorsal (thoracic) vertebra without mention of spinal cord injury. Approximately 50% of vertebroplasty inpatients and 38% of kyphoplasty inpatients were admitted from the emergency department. Mean and median length of stay were longer for vertebroplasty inpatients than for kyphoplasty inpatients. On-line Table 1 presents characteristics of the patients by procedure (vertebroplasty and kyphoplasty) and by setting of care (inpatient and outpatient).
The mean bed size of hospitals performing vertebroplasty was 460.9 ± 224.0 (n = 2330) for inpatients and 417.7 ± 198.4 (n = 1287) for outpatients. The mean bed size of hospitals performing kyphoplasty was 416.4 ± 206.1 (n = 4404) for inpatients and 425.9 ± 207.8 (n = 3714) for outpatients. Most hospitals were urban, and approximately two-thirds were nonteaching. A greater proportion of vertebroplasties and kyphoplasties were performed in hospitals in the Midwest. Approximately one-third of vertebroplasties in the data base were performed in hospitals in the Northeast. More than 70% of patients had traditional Medicare as their payer (Table 2).
Vertebroplasty and kyphoplasty hospital characteristics
Most vertebroplasties were performed by physicians with a specialty in radiology (70.7%) followed by orthopedics (9.9%), neurologic surgery (7.3%), and anesthesia (1.8%). Kyphoplasties were most commonly performed by physicians with a specialty in orthopedics (37.2%), followed by radiology (27.0%), neurologic surgery (26.1%), and anesthesia (1.9%).
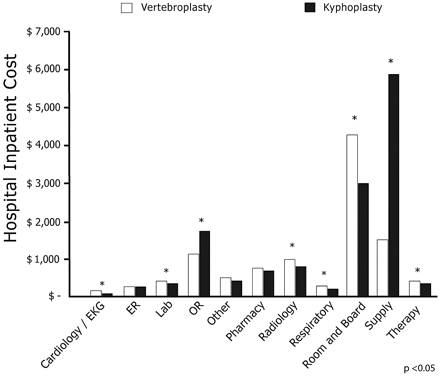
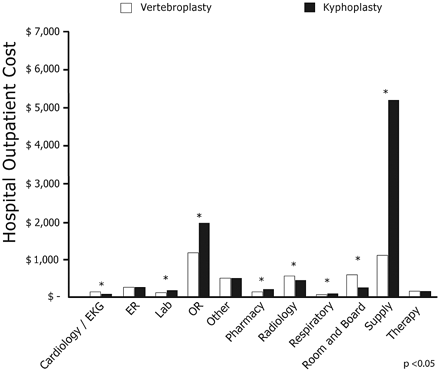
Mean total unadjusted inpatient costs were $9837 for vertebroplasty compared with $13,187 for kyphoplasty (P < .0001). Mean total unadjusted outpatient costs were $3319 for vertebroplasty compared with $8100 for kyphoplasty (P < .0001). Figures 2 and 3 present unadjusted costs by charge category for vertebroplasty and kyphoplasty inpatients and outpatients, respectively.
Unadjusted costs by charge category for vertebroplasty and kyphoplasty inpatients.
Unadjusted costs by charge category for vertebroplasty and kyphoplasty hospital outpatients.
Lower total costs for patients in the vertebroplasty cohort were largely due to differences in hospital supply and OR costs. Mean inpatient OR costs for vertebroplasty patients were $73.60 (anesthesia), $112.06 (recovery room), and $990.12 (surgery) compared with $172.16 (anesthesia), $257.47 (recovery room), and $1,471.49 (surgery) among kyphoplasty patients. Anesthesia and recovery room costs were 57% lower and surgery costs were 33% lower for vertebroplasty compared with kyphoplasty in the inpatient setting. In the outpatient setting, OR costs were $74.16 (anesthesia), $213.69 (recovery room), and $974.22 (surgery) for vertebroplasty patients compared with $182.98 (anesthesia), $289.35 (recovery room), and $1,520.24 (surgery) for kyphoplasty patients. These represent 60% lower anesthesia costs, 26% lower recovery room costs, and 33% lower surgery costs for vertebroplasty versus kyphoplasty in the outpatient setting. Radiology costs and room and board costs were higher with vertebroplasty but not sufficiently higher to offset lower costs in other hospital departments.
Adjustments to control for age, sex, admission status, and disease severity accentuated the differences in costs between the 2 cohorts. Mean total adjusted inpatient costs were $11 386 for vertebroplasty compared with $16 182 for kyphoplasty (P < .0001). Mean total adjusted outpatient costs were $2997 for vertebroplasty compared with $7010 for kyphoplasty (P < .0001). After adjustments for the same covariates, differences in inpatient length of stay between vertebroplasty and kyphoplasty were no longer evident (P = .4945).
Discussion
This study provides real-world evidence of significant cost differences between vertebroplasty and kyphoplasty for treatment of patients with painful VCFs. After adjusting for differences in patient baseline characteristics, mean total cost per inpatient procedure was $4796 lower among patients in the vertebroplasty cohort compared with those in the kyphoplasty cohort. For patients receiving outpatient procedures, mean adjusted total cost was $4013 lower among patients in the vertebroplasty group versus those in kyphoplasty group.
Lower hospital supply costs and OR costs contributed most to the total cost savings associated with vertebroplasty versus kyphoplasty. Within the OR, lower costs were driven by lower anesthesia, recovery room, and operating-time costs. Due to longer surgical duration as well as the use of larger gauge needles and a bone drill, kyphoplasty is more commonly performed with the patient under general anesthesia. In contrast, vertebroplasty is more commonly performed with the patient under moderate sedation at a lower cost. The potential for postanesthesia cognitive and physical impairment is also an important consideration in treatment planning for elderly patients with VCF, many of whom have cardiopulmonary comorbidities.
In 2009, there were approximately 80 000 VAP procedures among Medicare beneficiaries. Applying the setting of care distributions and costs observed in this study to this population allows estimation of the total costs that will be passed on by hospitals to the Medicare program through future reimbursement. If all of these procedures were kyphoplasties (setting-weighted average adjusted per-case cost of $11 986), total adjusted hospital costs would have been approximately $960 million versus $670 million if all were vertebroplasties (setting-weighted average adjusted per-case cost of $8401)—an annual difference of nearly $300 million.
These cost differences raise questions about the factors that influence physician selection of VAPs for treatment of VCFs. Historically, concerns about symptomatic cement leakage may have played a role in the selection of kyphoplasty over vertebroplasty, but newer vertebroplasty procedures that use high-viscosity cement have been shown to reduce cement leakage rates to those observed with balloon kyphoplasty.13 The low cost and short procedural time associated with vertebroplasty make the procedure an attractive choice for VCFs; however, some practitioners who do both procedures believe that vertebroplasty does not restore height to the same degree as kyphoplasty.14 Controversy surrounding the clinical implications of such differences have yet to be resolved.15
The challenge to both payers and health care providers is to maximize the net clinical benefit that patients receive from health care interventions, while minimizing total cost. The economic impact of both vertebroplasty and kyphoplasty should, therefore, be considered in the context of the ongoing debate surrounding the effectiveness of all treatment options for patients with VCFs. In addition to more clinical research that would shed additional light on variables related to natural history, regression to the mean, and nonspecific (placebo) effects of VAPs, more economic research that takes into account broader perspectives of these refractory patients is needed.
Our study differs from other published studies that have evaluated the economic consequences of vertebroplasty and kyphoplasty by considering the impact of patient characteristics on total hospital costs. The study by Lad et al (2009)10 was the only one comparing the economics of vertebroplasty and kyphoplasty, though this study did not account for differences in patient characteristics and relied on hospital charge data. Unlike charges, which include hospital mark-up, the costs used in our study were derived from hospital-specific cost information.16 Hospital costs are also a more accurate representation of payer financial burden, given that reimbursement rates commonly rely on historical cost data.
Our study has some limitations. Because patients were not randomized, results may be confounded by patient-specific variables that were not identified. We attempted to adjust for patient baseline differences, but our ability to control for differences between treatment groups was limited to the variables available in the Premier Perspective dataset. Another limitation is that patients included in the analysis may not be representative of all patients with VCF who receive VAPs. For example, we cannot draw conclusions about the relative economics of VAPs for specific subpopulations, including patients with VCFs from major trauma (who cannot be readily distinguished in the dataset from patients with VCFs from minor trauma) or those with cancer (who were excluded from this analysis). Additional study is warranted to better understand the economics of VAPs for these subpopulations. We also excluded patients who had multiple hospital admissions or outpatient visits for either vertebroplasty or kyphoplasty, because hospital costs for these patients may differ from those observed for patients in our study. Finally, we observed more inpatient than outpatient procedures in our analysis, which may not reflect national site-of-care distributions for these procedures.
The patients included in the Premier Perspective data base received treatment in >600 hospitals from all regions of the United States. However, Premier hospitals are predominantly small-to-midsize nonteaching facilities serving a largely urban patient population. Furthermore, the data used for this study were not specifically collected for research purposes. Procedure and diagnosis coding may not always be accurate or may be missing, though such errors would likely have a similar impact on each study cohort. Last, this study does not seek to address the postacute economics of VAPs, which warrant investigation in follow-up study. Despite these limitations, this study provides real-world evidence of the significant cost differences between vertebroplasty and kyphoplasty in the acute treatment of VCFs.
Conclusions
In the acute setting, vertebroplasty is cost-minimizing versus kyphoplasty for treatment of VCFs refractory to conservative medical management. Performing vertebroplasty versus kyphoplasty reduces hospital costs by nearly $5000 for inpatient procedures and by more than $4000 for outpatient procedures. These cost differences were observed despite older age and greater disease severity for inpatients in the vertebroplasty group. Further research is necessary to evaluate the long-term cost-effectiveness of treatment options for VCF.
Footnotes
This work was supported by DePuy Spine Inc.
-
Paper previously presented at: 16th Annual Meeting of the International Society for Pharmacoeconomics and Outcomes Research, May 15–18, 2010; Atlanta, Georgia.
-
Indicates article with supplemental on-line table.
Disclosures: Jason Lerner, Research Support (including provision of equipment or materials): DePuy Spine, Inc. Details: Employee. Luella Engelhart, Research Support (including provision of equipment or materials): DePuy Spine, Inc. Details: Employee. Chris M. Kozma, Research Support (including provision of equipment or materials): DePuy Spine, Inc. Details: Depuy Spine provides funding for data base research in spine-related diseases. Terra Slaton, Research Support (including provision of equipment or materials) DePuy Spine, Inc. Details: Consultant and programme. Natalie C. Edwards, Research Support (including provision of equipment and materials): DePuy Spine, Inc. Details: Research consultant. Gregory J. Lawler, Research support: DePuy Spine, Inc.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received August 8, 2010.
- Accepted after revision November 18, 2010.
- Copyright © American Society of Neuroradiology