Abstract
SUMMARY: The human HOXA1 mutation syndromes commonly present with abnormalities of the inner ear and ICAs. Previous cases describe varying degrees of hypoplasia or aplasia of the affected structures, often with asymmetric involvement. We present imaging findings documenting complete absence of the ICAs bilaterally with bilateral CLA, which, to our knowledge, has not been previously reported.
Abbreviations
- ABDS
- Athabascan brainstem dysgenesis syndrome
- BSAS
- Bosley-Salih-Alorainy syndrome
- CLA
- complete labyrinthine aplasia
- CN
- cranial nerve
- HOX
- homeobox
- ICA
- internal carotid artery
- MRA
- MR angiography
Two congenital syndromes with similar features, BSAS and ABDS, are recessive disorders linked to mutations of the HOXA1 gene (Online Mendelian Inheritance in Man, #601536). BSAS has been documented in consanguineous marriages in Saudi Arabian and in Turkish families.1–3 ABDS has been described in Native Americans of Athabascan (primarily Navajo and Apache) descent.3–5 We present a case of a 2-year-old Navajo girl with developmental delay, deafness, and horizontal gaze palsy, features of ABDS.
Case Report
A 2-year-old female patient of Navajo descent was assessed for developmental delay. The child was unable to speak or walk and had failed neonatal hearing screens. Brain stem auditory-evoked-response testing confirmed bilateral sensorineural deafness. She also exhibited impairment of conjugate horizontal eye movements and facial diplegia with fasciculations. There was no indication of cardiac anomaly or central hypoventilation.
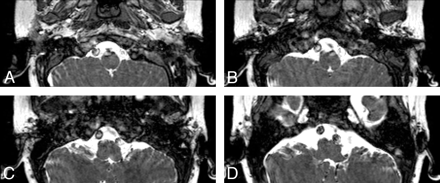
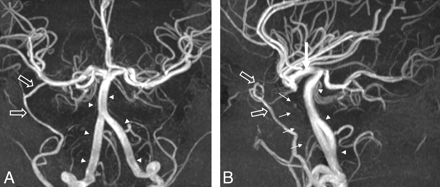
MR imaging of the brain demonstrated absent ICA flow voids and complete absence of the bilateral inner ear structures and internal auditory canals. The sixth-through-eighth cranial nerves could not be identified (Fig 1). MRA of the head confirmed bilateral absence of the ICA with markedly enlarged vertebral and basilar arteries supplying blood flow to the anterior and posterior circulations via an intact circle of Willis (Fig 2).
Balanced fast-field echo axial T2 images show complete lack of inner ear structures and internal auditory canals bilaterally.
3D time-of-flight maximum-intensity-projection images in anteroposterior and lateral projections demonstrate complete absence of the bilateral ICAs (small arrows). The vertebrobasilar system is enlarged (arrowheads) and supplies flow to the bilateral anterior cerebral arteries and middle cerebral arteries via enlarged posterior communicating arteries (large arrow). The right middle meningeal artery is enlarged (open arrows).
On the basis of findings of CLA, ICA aplasia, horizontal gaze abnormalities, and developmental delay in a Navajo child, the presumptive diagnosis of ABDS was made.
Discussion
The Navajo are a Native American tribe of Athabascan descent. Several recessive genetic disorders occur with increased frequency in Athabascan populations.5,6 In 1996 and 1997, Friedman et al4 presented 10 cases of ABDS, a congenital syndrome in Athabascan children featuring horizontal gaze abnormalities, deafness, developmental delay, and central hypoventilation. Variably present features included facial paresis, swallowing dysfunction, vocal cord paresis, seizures, and congenital cardiac anomalies. MRA in 3 ABDS cases revealed 2 children with unilateral hypoplasia or aplasia of the ICA. To our knowledge, these 13 cases are the only previously published reports of ABDS.3–5
On the basis of the number of cases identified and birth rates within studied populations, Erickson6 and Holve et al5 estimated the incidence of ABDS at 0.5–1 per 1000 live births on the White River Apache Reservation and 1 per 3000 live births in the Navajo population. If accurate, this finding suggests a carrier frequency similar to that of cystic fibrosis in whites. ABDS may represent a significantly under-recognized disorder among Athabascan Native Americans, raising questions of the possible benefit of genetic counseling.
In 2005, Tischfield et al1 observed a syndrome similar to ABDS in consanguineous Saudi Arabian and Turkish families. This syndrome, BSAS, has been described in 16 children. As in ABDS, horizontal gaze abnormalities, deafness, and developmental delay were common findings. Thirteen of the children with BSAS underwent neuroimaging studies; most exhibited ICA hypoplasia or aplasia. The only major feature of ABDS not found in BSAS was central hypoventilation.1–3 Tischfield et al and Bosley et al2 identified homozygosity for a mutation of the HOXA1 gene in all patients with BSAS. Eight children with ABDS, including 3 new cases, were subsequently studied and were also found to have homozygous HOXA1 mutations.
HOX genes are expressed in overlapping temporal patterns and spatial regions in the embryo and play an important role in determining cell identity along the cranial-caudal axis. HOXA1 is the first of the HOX genes expressed and is found in the most cephalad distribution. Two HOXA1−/− mouse models exhibited phenotypes similar to human HOXA1 syndromes, with the exception that cerebrovascular anomalies were not documented in mice.1 Thalidomide exposure between 20 and 24 days' gestation also causes anomalies resembling the HOXA1 mutation syndromes.2 Later exposure to thalidomide and mutations of HOX genes expressed later and more caudally both disrupt development of the extremities.1 These similarities suggest the teratogenic effects of thalidomide may represent a disruption of the complex HOX signaling cascade.2
Horizontal gaze palsies and other CN dysfunctions were commonly identified in patients with HOXA1 mutation syndromes. Horizontal gaze dysfunction was the most consistently identified abnormality, ranging from total horizontal gaze palsy to normal ocular motility in a few patients.3 HOXA1−/− mice also exhibited horizontal gaze palsies with absence of CN VI.2 Dysfunctions of CN VII–X were variably seen in patients with ABDS and BSAS. Facial paresis and deafness were common, while swallowing dysfunction and vocal cord paresis were less often documented.3 Our patient presented with intact vertical but restricted horizontal gaze, facial diplegia and fasciculations, and sensorineural deafness. CN VI–VIII could not be identified on MR imaging and are likely severely hypoplastic or absent.
Bilateral deafness was identified in most individuals with HOXA1 mutation syndromes and in all reported cases of ABDS.1–5 Imaging of the temporal bone has not been previously described in ABDS but has been reported for most patients with BSAS. Inner ear malformations ranged from CLA to minor cochlear hypoplasia. A few patients with BSAS were found to have normal inner ears and hearing. In the most striking malformations, unilateral CLA was identified with a common cavity deformity present contralaterally.3 Previous authors have described a similar wide spectrum of inner ear malformations in patients without the HOXA1 mutation. Most interesting, none of these patients exhibited ICA aplasia or any other features seen in ABDS.7 To our knowledge, the present case demonstrates the first known report of bilateral CLA in a patient with HOXA1 mutation syndrome.
Cerebrovascular anomalies were identified in more than half of the appropriately studied patients with HOXA1 mutation syndromes. Unilateral hypoplasia or aplasia of the ICA was most common, with bilateral ICA aplasia identified in 1 patient with BSAS.3 Collateral vasculature may fully compensate for ICA aplasia, but patients may be predisposed to aneurysm formation and cerebrovascular injury.8 MR imaging and MRA studies of our patient revealed bilateral ICA aplasia with markedly enlarged vertebral and basilar arteries supplying the anterior and middle cerebral vascular territories. To our knowledge, Figs 1 and 2 represent the first published neuroimaging studies in a case of ABDS.
Central hypoventilation is the only major feature that significantly differed between the HOXA1 mutation syndromes. No patient with BSAS was diagnosed with central hypoventilation, while reports of all 13 previously published cases of ABDS indicated that supplemental oxygen or mechanical ventilation was required.3,5 In all patients, hypoventilation was more severe during sleep, and in the least affected children, supplemental oxygen was only required at night.5 Because polysomnography was not conducted on the current patient, it is not possible to rule out mild central hypoventilation during sleep. However, pulse oximetry on room air was normal.
The present case exhibits the most extreme manifestations of ICA and inner ear abnormalities seen in ABDS, with complete bilateral aplasia of both. However, unlike every previously reported case of ABDS, the patient has no documented central hypoventilation. Considering the small number of patients previously studied, we suggest phenotypic variability is not yet fully appreciated in ABDS. The frequency of the disorder is also unclear, but preliminary estimates indicate it could be significantly under-recognized among Athabascan Native Americans. Increased awareness of this syndrome in conjunction with focused neuroimaging in the appropriate patient population is necessary to further elucidate the phenotype and prevalence of ABDS. Therefore, we believe all Native American children presenting with horizontal gaze palsies and sensorineural hearing deficits should undergo MR imaging with sequences optimized for the evaluation of inner ear structures, cranial nerves, and cerebral vasculature.
References
- Received July 20, 2009.
- Accepted after revision October 26, 2009.
- Copyright © American Society of Neuroradiology